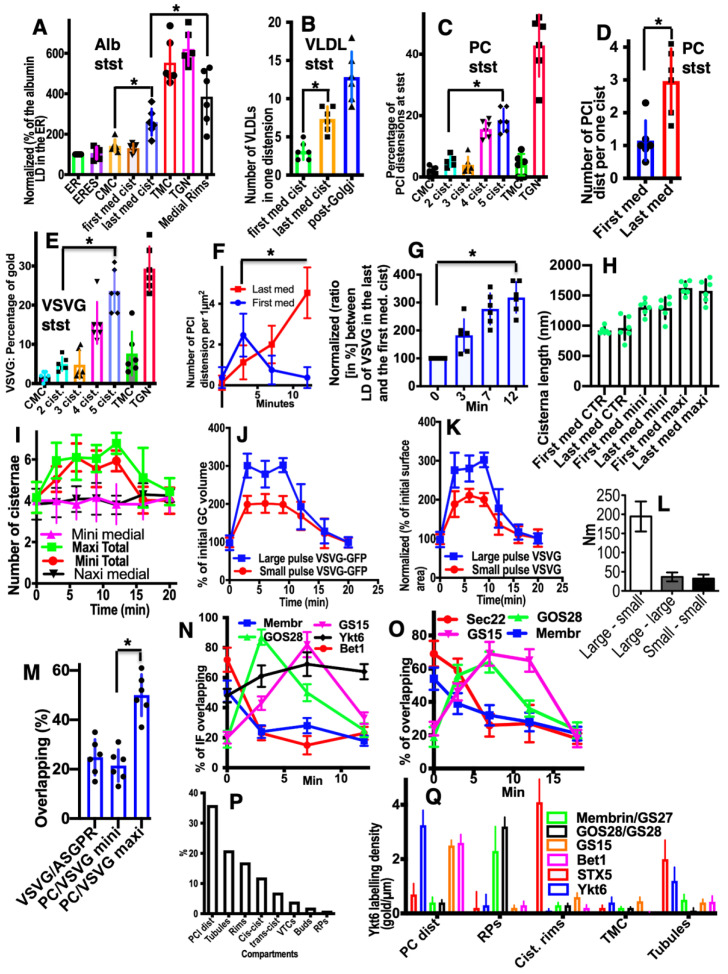
Figure 4.
Quantification of data. (A–E) Steady-state (stst). Normalised (versus the ER) gold labelling of albumin (A) and VSVG (E). Normalised (versus the first medial cisterna) number of VLDLs in one cisternal distension (B). Normalised number of procollagen I-containing distensions in one cisterna (C,D). (A) Gold labelling for albumin in the last medial cisterna and the trans-most cisterna is significantly higher than that in the first medial cisterna (p < 0.05). (B) The number of VLDLs in one cisternal distension of the last medial cisterna is significantly higher than that in the first medial cisterna (p < 0.05). (C) Distribution of PCI distension within the Golgi complex. Its proportion in the last medial cisterna is significantly higher than that in the first medial cisterna (p < 0.05). (D) The numerical density of PC distensions in the last medial cisterna is significantly higher than that in the first medial cisterna (p < 0.05). (E) Distribution of VSVG gold labelling within the Golgi complex. The numeric density of gold in the last medial cisterna is significantly higher than in the first medial cisterna (p < 0.05). (F) The mini-wave. The number of PCI distensions per 1 µm2 of cisterna. Their maximal number in the last medial cisterna is significantly higher than in the first medial cisterna (p < 0.05). (G) Labelling for VSVG becomes significantly enriched in the last medial cisterna (p < 0.05). (H) The length of the first and last medial cisternae do not differ both at steady-state (CTR) and during synchronous IGT (mini and maxi-waves). (I) During IGT (according to the mini-wave and maxi-wave protocols), the number of medial Golgi cisternae does not change, whereas two additional cisternae (cis-most and trans-most cisterna) are attached. (J,K) Dynamics of Golgi volume (J) and surface area of Golgi compartments (K) during the synchronous IGT of VSVG-GFP according to the mini-wave (red line) and maxi-wave (blue line) protocols. During IGT, the volume of the Golgi complex and the surface area of the Golgi compartments (K) significantly increased (p < 0.05), but this depended on the amount of cargo transported. (L) Distance between large (VSVG) and small (ManII) gold particles is significantly higher than between large gold particles or between small gold particles (p < 0.05). (M) Overlapping of immunofluorescence (IF) labelling between PC and VSVG when maxi-wave synchronisation was applied is significantly higher than when the mini-wave was used (p < 0.05). During IGT synchronised according to the cycloheximide (CHM)-15-CHM protocol, overlap between ASGPR and VSVG was low, similar to that after the mini-wave PCI and VSVG IGT. (N,O) Dynamics of co-localisation between PCI (N) and VSVG (O) with different Golgi SNAREs during the synchronised IGT, according to the CHM-15-CHM protocol. (N) Overlapping of IF between PCI and different Golgi SNAREs during IGT. PCI lost (p < 0.05) its co-localisation with Bet1 and then acquired co-localisation with GOS28 and GS15. (O) Overlapping of IF between VSVG and different Golgi SNAREs during IGT. VSVG lost (p < 0.05) its co-localisation with Sec22 and membrane and then acquired co-localisation with GOS28 and GS15. (P) distribution of immune-EM labelling for Ykt6 during IGT of PCI. Mini-wave. Ykt6 is depleted in round profiles (RPs) and enriched over PCI distensions (PCI dist.). (Q) Distribution of labelling of different Golgi SNAREs over the Golgi compartments at 4 min after release of the transport block of PCI. Mini-wave. Membranes of PCI distensions contain Ykt6, GS15 and Bet1. RPs are enriched in membrane and GOS28. Cisternal rims are enriched in syntaxin-5 (STX5). * p < 0.05.