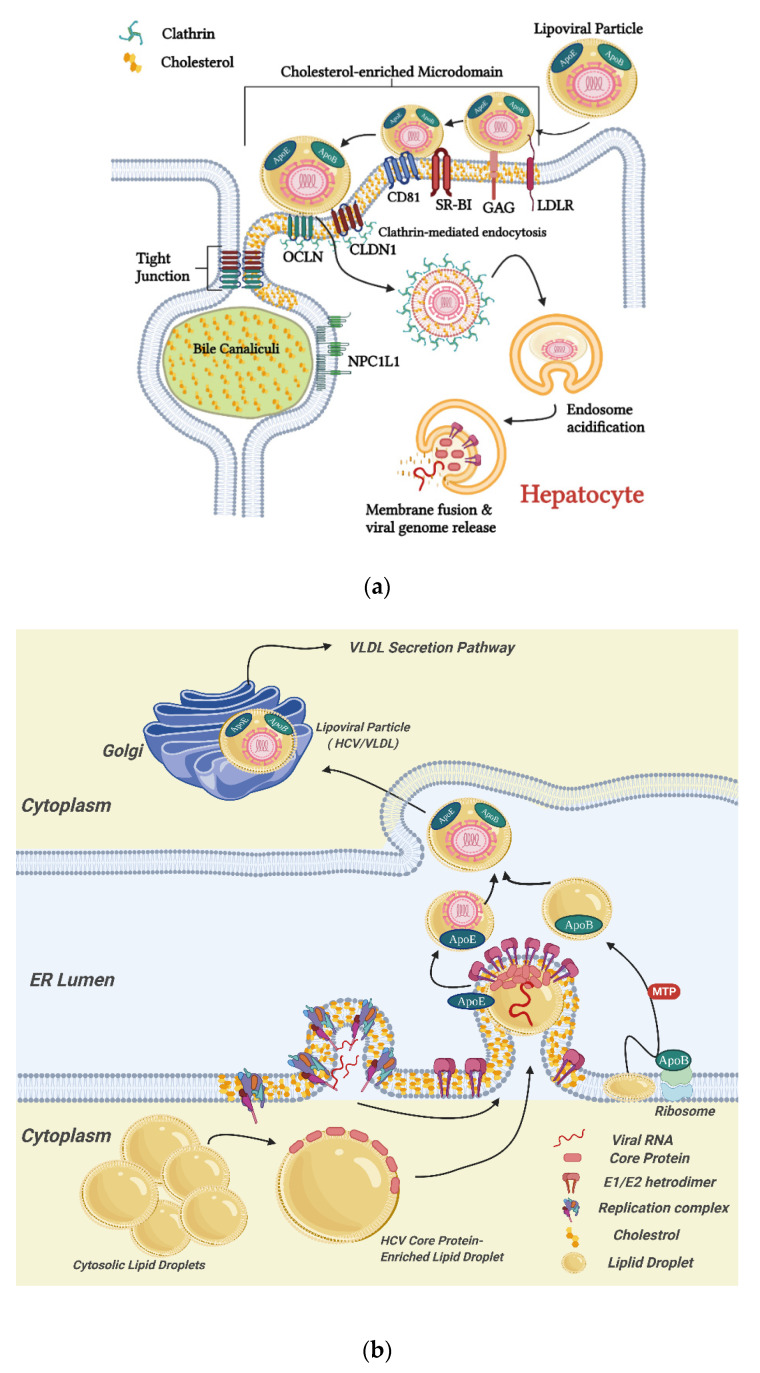
Figure 2.
Schematic diagrams of the HCV entry into hepatocyte (a) and the replication, assembly, and secretion of HCV (b). (a) HCV entry into hepatocytes is a sequential process in which multiple factors, including glycosaminoglycan (GAG), low-density lipoprotein receptor (LDLR), scavenger receptor class B type I (SR-BI), CD81, tight junction proteins, claudin-1 (CLDN1), and occludin (OCLN), as well as Niemann–Pick C1-like 1 (NPC1L1), are involved [73,87,88]. The process starts with the binding of the lipoviral particle (LVP) to GAG and LDLR, followed by the interactions with SR-BI, CD81, CLDN1, and OCLN, leading to viral internalization in cholesterol-enriched microdomains (lipid rafts) of the membranes via a clathrin-mediated endocytosis; (b) HCV viral proteins induce rearrangement of ER membranes and form replication complexes [15]. Localization of the HCV polyprotein to cholesterol-enriched membrane fractions is required for the polyprotein cleavage [89], and the replication complexes are associating with cholesterol-enriched domains [83]. New viral genomic RNA is synthesized by the replication complexes assembled by non-structural (NS) proteins of HCV, and the newly produced HCV core protein is translocated to LDs. Virion assembly occurs on core protein-enriched LDs and is associated by ApoE. Packaging of the capsid takes place with viral budding into the ER lumen at VLDL synthesis sites, mediated by microsomal triglyceride transfer protein (MTP). ApoB is lipidated by the MTP to generate VLDL precursors, which further fuse with ER luminal ApoE-bound LDs to form VLDL [87]. The matured HCV is associated with VLDL, in the form of LVP, and secreted through the VLDL secretion pathway. Graphics in this figure were created with BioRender.com (accessed on 26 February 2022).