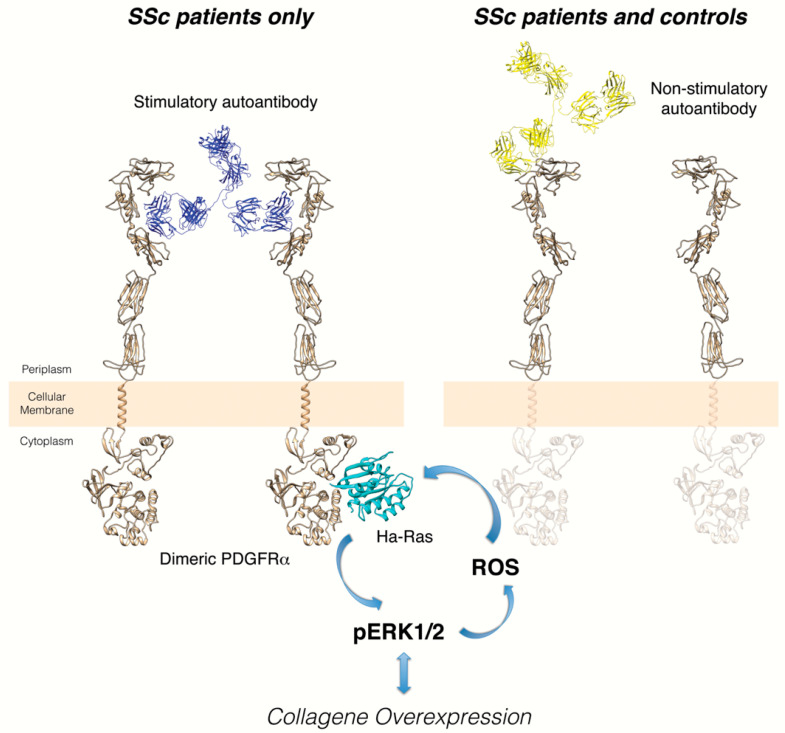
Figure 1.
Distinct epitopes of stimulatory and non-stimulatory anti-PDGFRα autoantibodies. Left cartoon: the interaction of stimulatory autoantibody cloned from memory B cells of a scleroderma patient and the discontinuous, conformational epitope identified on the extracellular PDGFRα domain induces the intracellular signaling activation and collagen gene overexpression. The identification of this binding region of PDGFRα provides a possible target for new therapeutic strategies to block excessive collagen synthesis under pathologic conditions such as SSc. Right cartoon: binding of non-stimulatory autoantibodies cloned from memory B cells of the same scleroderma patient to a single linear epitope of extracellular PDGFRα domain does not induce receptor dimerization/activation. This non-agonistic autoantibody could reflect the natural autoimmunity, already observed in many healthy individuals, without any clear role in physiology or disease.