Figure 1. PAX7::GFP MPC derived from hPSCs can form new muscle fibers and localize to the muscle stem cell niche in vivo.
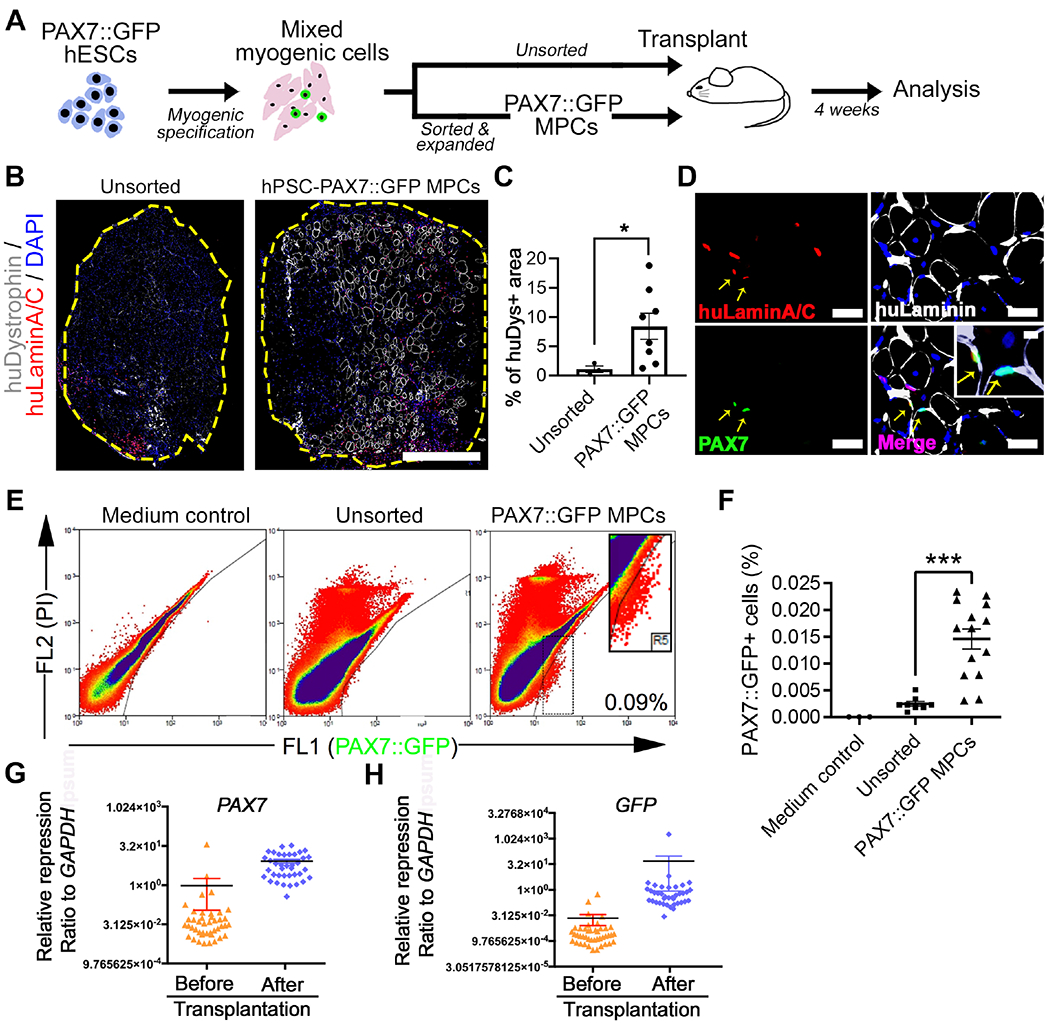
(A) Schematic of PAX7::GFP MPC derivation, cell transplantation, and downstream analysis. (B) Representative images of cross-section of tibialis anterior (TA) muscle of NSG mouse 4 weeks after transplantation of either sorted and expanded (PAX7::GFP MPC) or unsorted myogenic cells derived from PAX7::GFP hESCs. These cells participated in regeneration and formed new muscle fibers (labeled by huDystrophin) that have donor cell origin. All donor originated cell nuclei were labeled with huLaminA/C+ antibody (red). Edge of TA was highlighted by yellow. Scale bar=500μm (C) Quantification of percentage of huDytrophin+ fibers area against the entire TA area (n(Unsorted)=3, n(Expanded)=8; Student’s t-test, *p<0.05). (D) Images of sectioned TAs 4 weeks after the PAX7::GFP MPC transplantation.PAX7::GFP MPC progenies could be identified by double labeling of huLaminA/C (red) and PAX7 (green) proteins. They locate under basal Lamina (yellow arrow). Scale bar=100μm (E) FACS plot of ‘in vivo PAX7::GFP+ cells’ isolated 4 weeks after either being sorted and expanded or unsorted. TAs injected with vector medium was used as control. (F) Quantification of PAX7::GFP+ cells as a proportion of all isolated mononucleated cells from a single TA (n(Control)=4, n(Sorted & expanded)=14, n(Unsorted)=8; One-way ANOVA, *p<0.001). (G-H) Single cell Q-PCR showing quiescent muscle stem cell markers (PAX7) and confirmative marker GFP were expressed at higher level in in vivo PAX7::GFP+ cells compared with in vitro PAX7::GFP+ cells (n=48; Student’s t-test, *p<0.05).Result=Mean±SEM.
