FIGURE 1.
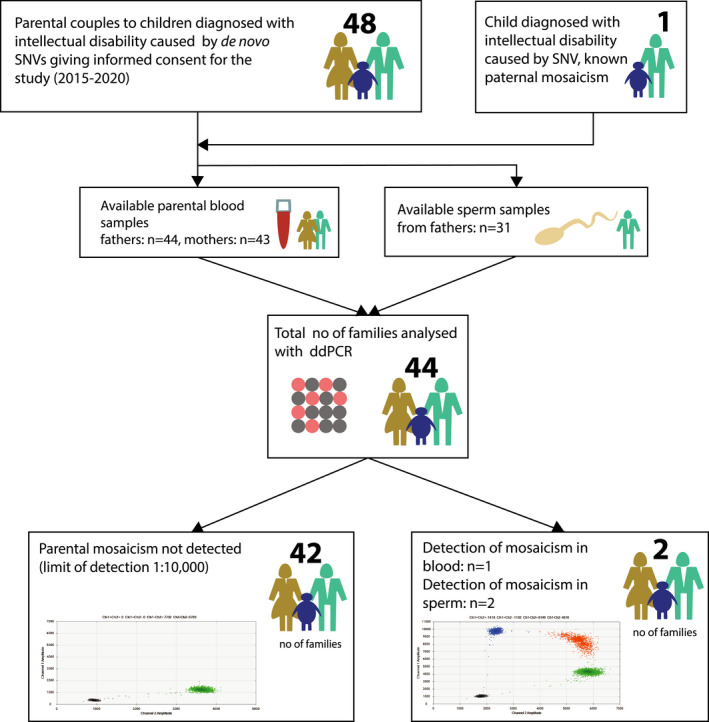
Overview of the study. We offered parents to children diagnosed with a genetic syndrome including intellectual disability due to de novo disease‐causing SNVs for participation in this cohort. All patients were initially referred for clinical diagnostic testing with trio whole‐genome sequencing/whole‐exome sequencing at the Department of Clinical Genetics of Karolinska university hospital, Stockholm, Sweden. After signing written consent, a test kit was sent home to fathers to provide sperm sample. Blood‐derived DNA was available prior to the study at the Karolinska university laboratory. Four families were excluded due to not sending in sperm sample (n = 2), lack of availability to parental blood samples and/or positive control in the family (n = 2). ddPCR, droplet digital PCR; SNV, single nucleotide variant
