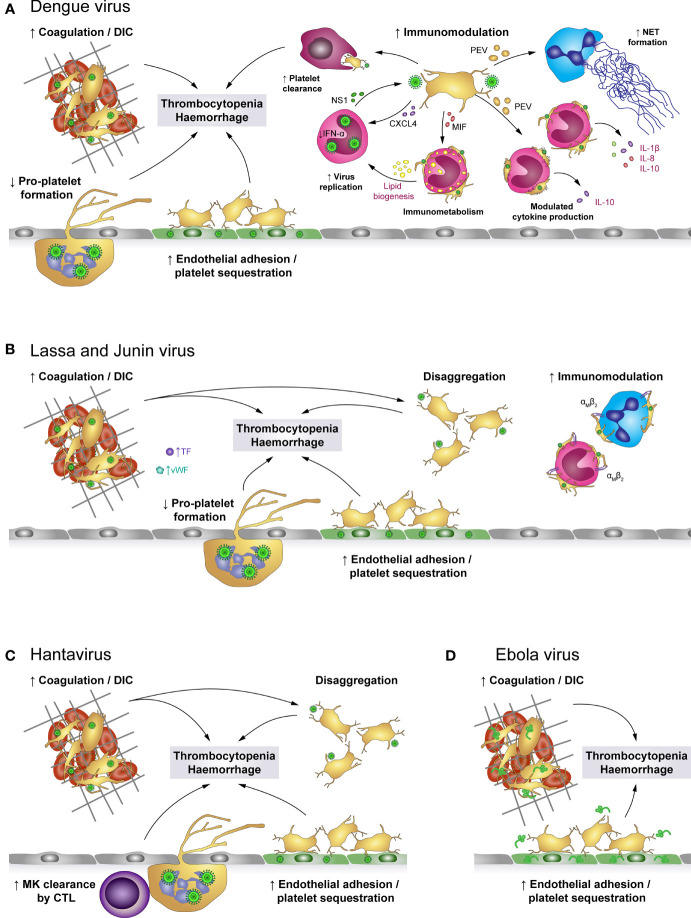
Figure 4.
The role of platelets in viral haemorrhagic fevers (VHF). (A) Dengue virus activates platelets and promotes the development of disseminated intravascular coagulation (DIC), leading to platelet consumption. Additionally, activated platelets adhere to infected endothelial cells and infected megakaryocytes (MK) show impaired pro-platelet formation. Together with macrophage-mediated clearance of virus-activated platelets, these mechanisms lead to thrombocytopenia and enhanced haemorrhagic complications. In addition, virus-stimulated platelets bind to leukocytes or release soluble factors to modulate immune responses such as NET formation as well as production of a specific cytokine profile, depending on whether interacting platelets are activated or apoptotic. Platelet-derived factors also promote virus replication in monocytes which is further fostered by modulated immunometabolism and platelet-induced lipid biogenesis. (B) Infection with Lassa or Junin virus is associated with elevated levels of von Willebrand factor (vWF) and tissue factor (TF) which contribute to platelet activation and development of DIC. While activated platelets readily bind to monocytes and neutrophils via their αMβ2 receptor, they are unable to maintain stable platelet-platelet interaction and rapidly disaggregate. Further, virus infection augments endothelial platelet adhesion and sequestration and limits pro-platelet formation of MKs. Thereby, infection with Lassa or Junin virus leads to thrombocytopenia and increases bleeding risk. (C) Hantavirus readily infects endothelial cells which promotes endothelial platelet adhesion and sequestration from the circulation. While infected megakaryocytes display no differentiation dysfunction, they are cleared by cytotoxic T lymphocytes (CTL). Systemic infection triggers a pro-coagulatory state which may exacerbate to DIC. Nevertheless, platelets show impaired capacity to form stable aggregates. Hantavirus thus causes thrombocytopenia and haemorrhagic complications by affecting key mediators of haemostasis. (D) Infection with Ebola virus is associated with systemic coagulation induction and risk for DIC, which leads to platelet consumption. However, platelets may also be sequestrated due to enhanced adhesion to the infected endothelium. Thereby, Ebola virus causes thrombocytopenia and haemorrhagic complications. CTL, Cytotoxic T cell; CXCL4, chemokine (C-X-C motif) ligand 4; DIC, Disseminated intravascular coagulation; IFN-α: Interferon α; IL, Interleukin; MIF, Macrophage migration inhibitory factor; MK, Megakaryocytes; NET, Neutrophil extracellular trap; NS1, Non-structural protein 1; PEV, Platelet-derived extracellular vesicle; TF, Tissue factor; VHF, Viral haemorrhagic fevers; vWF, von Willebrand factor.