Abstract
A primary cilium, a hair-like protrusion of the plasma membrane, is a pivotal organelle for sensing external environmental signals and transducing intracellular signaling. An interesting linkage between cilia and obesity has been revealed by studies of the human genetic ciliopathies Bardet-Biedl syndrome and Alström syndrome, in which obesity is a principal manifestation. Mouse models of cell type-specific cilia dysgenesis have subsequently demonstrated that ciliary defects restricted to specific hypothalamic neurons are sufficient to induce obesity and hyperphagia. A potential mechanism underlying hypothalamic neuron cilia-related obesity is impaired ciliary localization of G protein-coupled receptors involved in the regulation of appetite and energy metabolism. A well-studied example of this is melanocortin 4 receptor (MC4R), mutations in which are the most common cause of human monogenic obesity. In the paraventricular hypothalamus neurons, a blockade of ciliary trafficking of MC4R as well as its downstream ciliary signaling leads to hyperphagia and weight gain. Another potential mechanism is reduced leptin signaling in hypothalamic neurons with defective cilia. Leptin receptors traffic to the periciliary area upon leptin stimulation. Moreover, defects in cilia formation hamper leptin signaling and actions in both developing and differentiated hypothalamic neurons. The list of obesity-linked ciliary proteins is expending and this supports a tight association between cilia and obesity. This article provides a brief review on the mechanism of how ciliary defects in hypothalamic neurons facilitate obesity.
Keywords: ciliopathy, G protein-coupled receptor, hypothalamus, leptin, obesity, primary cilia
INTRODUCTION
Primary cilia are non-motile hair-like structures extruding from the cell surfaces that comprise a 9+0 tubulin-based cytoskeleton core called axoneme and a specialized plasma membrane covering the axoneme (Singla and Reiter, 2006). Primary cilia are assembled from a basal body derived from the mother centriole during the G0 or G1 phase of the cell cycle (Sánchez and Dynlacht, 2016). For cilia growth, ciliary membranes and axonemal proteins are synthesized in the cytoplasmic endoplasmic reticulum-Golgi and transported to the basal body area and then to the ciliary tip. The transport of ciliary proteins is mediated by the intraflagellar transport (IFT) machinery, which is composed of motor proteins (kinesin or dynein) and cargo IFT proteins (Ishikawa and Marshall, 2011; Rosenbaum and Witman, 2002).
Ciliary membranes express a number of signaling receptors, especially G protein-coupled receptors (GPCRs), and signaling molecules as well as ion channels, which is compatible with their functioning as a sensory organelle (Anvarian et al., 2019; Schou et al., 2015). Moreover, the primary cilia in epithelial cells and sensory neurons play an indispensable role in sensing external environments, including light, sound, olfactory signals, chemicals, temperature, mechanical force, flow of body fluids, and among others (Berbari et al., 2009; Singla and Reiter, 2006). Furthermore, primary cilia serve as a platform for cellular signal transduction. Multiple signaling pathways (such as sonic hedgehog, Wnt, PDGF, LKB1-AMPK, autophagy, etc.) are transduced via the primary cilia or are modulated by them (Anvarian et al., 2019; Aznar and Billaud, 2010; Pampliega et al., 2013; Song et al., 2018). A substantial body of evidence now indicates that ciliary defects are closely linked to the development of various causes of obesity. Here, we briefly illustrate how defects in cilia or cilia-related molecules promote obesity, with an emphasis on the central mechanisms involved.
OBESITY IN HUMAN GENETIC CILIOPATHY
The association between primary cilia and obesity stems from observations in human genetic ciliopathies such as the Bardet-Biedl syndrome (BBS) and Alström syndrome (ALMS). BBS patients commonly manifest obesity along with intellectual impairment, renal abnormalities, polydactyly, retinal degeneration, and hypogenitalism (Forsythe and Beales, 2013). These patients are typically born with a normal weight but 90% of cases rapidly gain weight in the first year of life. In addition, type 2 diabetes affects about 45% of BBS patients. To date, mutations in more than 20 genes (BBS1BBS22) have been linked to BBS (Pomeroy et al., 2021). Consistently, Bbs2-/-, Bbs4-/-, and Bbs6-/ - mice develop obesity (Rahmouni et al., 2008).
The molecular basis for the onset of obesity in BBS has been the subject of considerable study. A protein complex comprising BBS proteins, the BBSome, has been shown to work as an adaptor of the IFT complex (Liu and Lechtreck, 2018), and it has been suggested that BBS proteins may also mediate the ciliary transport/localization of molecules that are critical for body weight control. In this event, any defects in BBS proteins would likely impair the cilia-mediated functions of those molecules. A promising candidate as a cilia-related body weight regulator is neuropeptide Y receptor type 2 (Y2R), a known receptor of the gut-released anorexigenic peptide PYY3-36 and that localized on the cilia of hypothalamic neurons (Loktev and Jackson, 2013). Bbip10-/- mice, which have a defective BBSome structure, display a decreased ciliary localization of Y2R, blunted anorexigenic effects of PYY3-36, and weight gain (Loktev and Jackson, 2013). Another candidate is the melanin concentrating hormone receptor 1 (MCHR1), which binds the orexigenic neuropeptide MCH (Qu et al., 1996). MCHR1 is also localized on hypothalamic neuron cilia (Berbari et al., 2008). In Bbs2- and Bbs4-depleted cells, MCHR1 fails to localize to the cilia and instead accumulates in cytoplasmic puncta (Berbari et al., 2008). Hence, BBS mutations may hamper the functions of MCHR1 that take place on the cilia. However, the contribution of abnormal MCHR1 signaling to ciliopathy-related obesity is uncertain as Mchr1-/- mice exhibit a lean phenotype (Shimada et al., 1998). The association between BBS proteins and leptin signaling has drawn interest from many researchers (Berbari et al., 2013; Guo et al., 2016; Rahmouni et al., 2008; Seo et al., 2009) and will be discussed below in the section entitled “Cilia and BBS proteins in hypothalamic leptin signaling”.
ALMS is a rare autosomal recessive disorder caused by ALMS gene mutations and is characterized by vision and hearing abnormalities, childhood obesity and type 2 diabetes mellitus, hypogonadism, and slowly progressive kidney dysfunction (Álvarez-Satta et al., 2015). Alms1 mutant mice recapitulated the metabolic phenotype of ALMS (obesity, adipocyte hypertrophy, insulin resistance and hyperglycemia) (Collin et al., 2005), which was found to be rescued by reactivation of the Alms gene in adipocytes (Kang, 2021). ALMS1 localizes to the centriole and ciliary/basal body protein and is thought to regulate adipocyte differentiation and metabolism (Hearn et al., 2005). Interestingly, localization of ALMS1 at the base of cilia is also found in hypothalamic neurons. Moreover, Alms1 mutant mouse exhibits a significant loss of primary cilia in the hypothalamus (Heydet et al., 2013). Therefore, ALMS1 may regulate body weight through its action on hypothalamic neuron cilia.
Other obesity-linked monogenic ciliopathies include a syndrome of mental retardation, truncal obesity, retinal dystrophy and micropenis (MORM) induced by ciliary lipid phosphatase inositol polyphosphate-5-phosphatase E (INPP5E) mutations (Jacoby et al., 2009), and a syndrome of morbid obesity and spermatogenic failure (MOSPGF) caused by a homozygous mutation in CEP19 (Shalata et al., 2013). CEP19 localizes to the basal body to facilitate ciliogenesis and mediate the ciliary trafficking of GPCRs. Cep19-/- mice recapitulated these human phenotypes, including hyperphagia and an increased fat mass (Shalata et al., 2013).
HYPOTHALAMIC NEURONAL CILIA AND OBESITY
Almost every cell type contains primary cilia that manifest either transiently or permanently. An important question that arose from this was the nature of the cell types that played a major role in defective cilia-induced obesity. The answer subsequently came from mouse models of defective ciliogenesis that were generated by knocking out genes encoding IFT components (Ift88 and Kif3a) (Lechtreck, 2015; Lee et al., 2020). In these experimental mice, the adult-onset global ablation of Ift88/Tg737 or Kif3a led to increase in food intake, body weight, fat mass, hepatic lipid accumulation, and also elevated blood glucose and insulin levels (Davenport et al., 2007). In these animal models, increased food intake appeared to be a major driver of obesity as inhibition of hyperphagia by pair-feeding to wild type mice blunted their obesity phenotype (Davenport et al., 2007). As food intake is known to be regulated by the neurons in the central nervous system (CNS), the neuron-specific cilia dysgenesis model was generated to reproduce the hyperphagic obesity phenotype (Davenport et al., 2007). Based on these data, it has been proposed that abnormal cilia formation in CNS neurons may lead to obesity development.
The hypothalamus, particularly the hypothalamic arcuate nucleus (ARH), is a critical area with regard to the regulation of food intake and energy balance (Roh et al., 2016). Proopiomelanocortin (POMC) neurons in this area represent the neurons with anti-obesity actions (Morton et al., 2006; Schwartz et al., 2000). POMC neurons release α-melanocyte stimulating hormone (αMSH) which suppresses food intake through agonistic activity at the melanocortin 4 receptor (MC4R) (Ollmann et al., 1997). A POMC neuron-specific inhibition of ciliogenesis was reported to cause obesity when it was introduced during the mid-embryonic and early postnatal periods whereas it did not alter body weight when introduced in adulthood (Lee et al., 2020). In terms of the mechanism, the absence of functioning cilia in developing POMC neurons impairs embryonic neurogenesis and postnatal circuit organization, suggesting a critical role of primary cilia in the normal development of these neurons. The ventromedial hypothalamus (VMH) is another important area for body weight control. Mice with cilia dysgenesis in VMH steroidogenic factor-1 (SF1)-expressing neurons also acquire the obesity phenotype owing to an increase in food intake and a decrease in energy expenditure and brown adipose tissue thermogenesis (Sun et al., 2021). The hypothalamic paraventricular nucleus (PVH) also has a critical involvement in the regulation of both body weight and food intake (Roh et al., 2016). Loss of cilia in the MC4R-expressing PVH neurons causes obesity, hyperphagia, and increased body lengths (Siljee et al., 2018). Hence, studies using mouse models of ciliary dysgenesis have demonstrated that defective ciliogenesis in specific hypothalamic neuronal populations causes obesity and hyperphagia.
Conversely, obesity by itself appears to influence cilia formation in hypothalamic neurons. In adulthood, most of these neurons are terminally differentiated and have a single primary cilium on the soma surface. Hypothalamic cilia lengths vary from less than 2 μm to more than 10 μm, with average lengths of 5-6 μm (Han et al., 2014). Notably, the hypothalamic cilia lengths are shorter in obese conditions, with the lengths in the ARH and VMH found to be 30% shorter in obese mice than in age-matched lean controls (Han et al., 2014). More interestingly, maternal HFD feeding during the lactation period dampens ciliogenesis in the offspring’s hypothalamus (Lee et al., 2020). The obesity-associated short cilia phenotype appears to be related to reduced leptin signaling. In terms of the molecular mechanism, leptin promotes cilia growth in hypothalamic neurons via the PTEN/GSK3β signaling-dependent stimulation of IFT protein transcription and actin depolymerization, both of which are critical steps in ciliogenesis (Han et al., 2014; Kang et al., 2015). An obesity-associated short cilia phenotype has not been observed in other brain areas (Han et al., 2014; Lee et al., 2020). Taken together, a bidirectional regulatory process exists between ciliogenesis and obesity in hypothalamic neurons.
CILIA AND BBS PROTEINS IN HYPOTHALAMIC LEPTIN SIGNALING
Leptin is the most important hormone for preventing obesity and is released by the white adipose tissue in proportion to the fat mass (Frederich et al., 1995). Leptin controls food intake and energy expenditure by acting on the hypothalamus (Halaas et al., 1995). In hypothalamic leptin-target neurons, leptin exerts its signaling effects through the leptin receptor (LepRb) and via downstream signaling pathways including JAK2-STAT3 (Kwon et al., 2016). Several lines of evidence have confirmed that functional cilia are necessary for leptin signaling in the hypothalamus. The acute inhibition of ciliogenesis in the mediobasal hypothalamus in adult mice through the microinjection of small inhibitory RNAs specific to Kif3a and Ift88 was shown to mitigate the effects of leptin administration on food intake and energy expenditure, as well as on STAT3 activation (Han et al., 2014). Mice lacking cilia in SF1 neurons were reported to display increased blood leptin levels (hyperleptinemia), indicative of leptin resistance, and a blunted anorectic response and reduced STAT3 signaling upon leptin injection (Sun et al., 2021). We have also recently reported that the leptin-promoted circuit organization of POMC neurons is dependent on primary cilia (Lee et al., 2020).
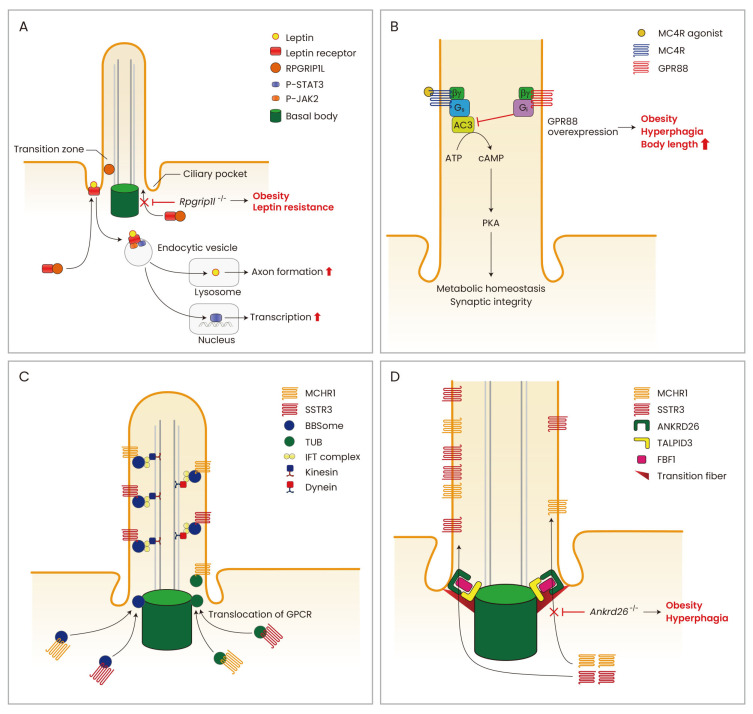
Determining the mechanism by which the primary cilia transduce leptin signaling in hypothalamic neurons was the core aim of many studies. The ciliary localization of LepRb has not been demonstrated yet and this receptor has only so far been detected around the basal body at the base of the cilium (Han et al., 2014; Stratigopoulos et al., 2014). Hence, leptin signal transduction has been speculated to take place in the periciliary area rather than on the cilium itself. Intriguingly, leptin treatments have been found to trigger the periciliary trafficking of LepRb and the inhibition of this process may disrupt leptin signaling (Stratigopoulos et al., 2014). For example, retinitis pigmentosa GTPase regulator-interacting protein-1 like (RPGLIP1L), a ciliary transition zone protein, was reported to mediate the periciliary trafficking of LepRb (Fig. 1A). Rpgrip1l+/- mice showed impaired localization of LepRb near the basal area of the cilium in the hypothalamic neurons, reduced leptin responses, increased fat mass and hyperphagia. In humans, single nucleotide polymorphisms (SNPs) in the first intron of the fat mass and obesity-associated (FTO) gene are closely associated with an increased risk of common obesity (Loos and Yeo, 2014). Interestingly in this regard, RPGLIP1L is a vicinal gene of FTO, and the expression of both genes is coregulated by an overlapping promoter region in the first intron of FTO (Stratigopoulos et al., 2016). Human subjects carrying obesity-related FTO SNPs (rs8050136, rs1421085) show reduced RPGRIP1Ll and FTO expression (Stratigopoulos et al., 2016). It is thus plausible that the development of obesity in FTO SNP carriers may be attributable to a reduced RPGRIP1L expression and the resultant impaired periciliary trafficking of LepRb in the hypothalamic neurons.
Fig. 1. Several models of cilia and cilia-related molecules in the hypothalamic neurons.
(A) Leptin signaling and cilia. In the hypothalamic neurons, functioning leptin receptors (LepRb) traffic to the periciliary area upon leptin stimulation and this process is mediated by the ciliary transition zone protein RPGRIP1L. The leptin-LepRb complex then undergoes endocytosis to stimulate STAT3-medited transcriptional regulation. Leptin also promotes to lysosomal proteolysis and axonal projection via cilia-dependent mechanisms. (B) MC4R signaling and cilia. The human monogenic obesity gene MC4R localizes at the cilia of hypothalamic PVH neurons. Ciliary adenylyl cyclase 3 (ADCY3)-cAMP signaling is critical for MC4R signaling as its blockade induces hyperphagia and weight gain. (C) BBSome/TUB and cilia. The obesity-related genes BBSome and TUB engage in the ciliary trafficking of GPCRs implicated in the control of feeding behavior and energy metabolism. (D) ANKRD26 and ciliary gating. The cilia transition fiber protein ANKRD26 allows the ciliary gating of GPCRs. ANKRD26 comprises this gate with TALPID3 and FBF1 and this gating complex facilitates ciliary GPCR import. A lack of Ankrd26 induces severe obesity and hyperphagia due to the reduced ciliary expression of MC4R and other GPCRs.
The relationship between BBS proteins and leptin signaling has also been studied by several research groups but the results seem to be somewhat contradictory. Bbs2-/-, Bbs4-/-, and Bbs6-/- mice and neuronal Bbs1-deficient mice show hyperleptinemia and blunted leptin responses (Rahmouni et al., 2008). It was thus suggested that impaired hypothalamic leptin signaling contributes to the development of obesity in BBS mouse models. In sharp contrast, other groups have reported that Bbs4-/- and Bbs6-/- mice show a normal leptin response under pre-obese and food-restricted conditions (Berbari et al., 2013). They contended that the leptin resistance observed in obese Bbs4-/- and Bbs6-/- mice is secondary, rather than a cause of, obesity. Another recent study has demonstrated that a knockdown of Bbs1 and Bbs2 reduces LepRb trafficking to the plasma membrane (Guo et al., 2016). This effect seemed to be cilia-independent as an Ift88 knockdown did not affect this trafficking. The crucial and cilia-independent role of BBS1 in leptin signaling was further reinforced by findings that a depletion of Bbs1 in LepRb-expressing neurons caused severe obesity, hyperphagia and leptin resistance, whereas cilia dysgenesis in the same neural population induced mild obesity without hyperphagia or leptin resistance (Guo et al., 2016).
In addition to LepRb, BBS1 also mediates the transport of the serotonin receptor 5-HT2C to the plasma membrane, especially in POMC neurons (Guo et al., 2019). Hence, Bbs1-depleted cells showed attenuated 5-HT2C expression on the cell surface and reduced 5-HT2C signaling in response to the appetite-suppressing drug lorcaserin, a 5-HT2C agonist. Moreover, Bbs1 deletion in both hypothalamic POMC and AgRP neurons leads to obesity, indicating the importance of the BBSome in these neurons in weight control (Guo et al., 2019). Further studies are needed to clarify the relative contribution of the cilia-related and -unrelated roles of BBSome in body weight maintenance.
OTHER OBESITY-LINKED CILIARY PROTEINS
AC3/ADCY3, encoded by the Adcy3 gene, is a cilia-abundant subtype of adenylyl cyclase that serves as a critical downstream effector of Gs-coupled GPCRs by producing cAMP (Qiu et al., 2016). AC3 immunostaining is commonly used for probing hypothalamic neuron cilia (Han et al., 2014; Wang et al., 2009; 2011). Human genetic studies have revealed a strong association between ADCY3 genetic variations and obesity in both adults and children (Grarup et al., 2018; Stergiakouli et al., 2014). Moreover, a recent study has reported homozygous loss-of-function mutations in the ADCY3 gene in 4 consanguineous Pakistani families with severe monogenic obesity and compound-heterozygous mutations of this gene in a severely obese child of European-American descent (Saeed et al., 2018). Consistently, Adcy3-/- mice exhibit adult-onset severe obesity, with the adult males found to be about 40% heavier than their wild type counterparts, and the females 70% heavier. Obesity in this animal model was shown to be related to increased food intake and decreased locomotor activity (Wang et al., 2009). Another study demonstrated that mice with an Adcy3 haploinsufficiency are prone to diet-induced obesity with no change in food intake and reduced thermogenesis (Tong et al., 2016).
In line with the aforementioned evidence, a gain-of-function mutation in Adcy3 was shown to induce resistance to diet-induced obesity in mice (Pitman et al., 2014). AC3 is highly expressed in the hypothalamic regions involved in body weight control, whereas brown and white adipose tissues have little AC3 expression (Wang et al., 2009). Adcy3-/- mice show reduced adenylyl cyclase activity in the hypothalamus but this activity is normal in the adipose tissue (Wang et al., 2009). These findings suggest that the adequate expression of AC3 in the hypothalamus is crucial for preventing obesity. In support of this notion, the viral-mediated depletion of Adcy3 in the mouse VMH was reported to cause increased fat mass and hyperphagia (Cao et al., 2016). Another study found that the lack of Adcy3 did not induce structural changes in the primary cilia (Wang et al., 2009) and it may therefore specifically disrupt GPCR-cAMP signaling in the cilia.
MC4R is a Gs-coupled GPCR that is critically linked to body weight homeostasis in mammals (Krashes et al., 2016). MC4R mutations are the most common cause (3%-5%) of monogenic obesity in humans (Bromberg et al., 2009; Lubrano-Berthelier et al., 2006; Siljee et al., 2018). A recent report has identified MC4R as an AC3-interacting receptor at the primary cilium (Siljee et al., 2018; Wang et al., 2021). In the PVH neurons, MC4R colocalizes with AC3 at the cilium and this ability is markedly reduced in human obesity-associated MC4R mutants (p.P230L and p.R236C) (Siljee et al., 2018) (Fig. 1B). Moreover, food intake and body weight gain are increased when ciliary AC3 signaling is inhibited by the overexpression of ciliary Gi-coupled GPCR GPR88 in either Sim1- or MC4R-expressing PVH neurons (Siljee et al., 2018). MC4R agonist (MTII)-induced anorexia is largely dependent on cilia and ciliary AC3 signaling in PVH neurons (Wang et al., 2021). These findings have provided important molecular insights into the intimate association among MC4R, cilia-mediated signaling, and obesity.
TUB, a tubby homolog in human, is a member of the tubby-like protein (TULP) family that is predominantly expressed in neurons (He et al., 2000). Tubby mice with naturally-occurring Tub mutations display maturity-onset obesity, insulin resistance and sensory deficits which resemble the BBS and ALMS phenotypes in human (Noben-Trauth et al., 1996). A loss of Tub disrupts the ciliary transport of specific GPCRs such as rhodopsin, Mchr1 and Sstr3 (Fig. 1C). However, it was found not to induce generalized defects in ciliogenesis or protein trafficking (Sun et al., 2012). Polymorphisms in TUB were shown previously to be associated with the body mass index and carbohydrate intake in middle-aged women (van Vliet-Ostaptchouk et al., 2008). Moreover, homozygous mutations in TUB were found to be associated with retinal dystrophy and obesity syndrome in humans (Borman et al., 2014). TUB is also expressed in adipose tissue and its reduced expression in the adipose tissue of severely-obese human subjects has been described (Nies et al., 2018).
The ankyrin repeat domain 26 (ANKRD26) protein is localized in the ciliary transition fibers (Yan et al., 2020) and its gene is located on chromosome 10p12, a locus that has been associated with the genetic form of obesity in humans (Dong et al., 2005). In line with this, Ankrd26-/- mice present with hyperphagia, severe obesity, gigantism and elevated serum leptin levels (Bera et al., 2008). Ankrd26 was widely expressed in the brain areas with robust expression in the ARH and PVH and brainstem solitary tract nucleus (NTS) (Acs et al., 2015), all of which is critically related of feeding regulation. Hyperphagia and obesity in Ankrd26-/- mice were related to a reduction in MC4R expression and other ciliary GPCRs such as MCHR1 and SSTR3 especially in the PVH whereas they showed normal leptin signaling in the ARH neurons. Interestingly, a recent paper has shown that in Caenorhabditis elegans and mammalian cells, an interaction of ANKRD26 and Joubert syndrome protein TALPID3 is critical for the recruitment of FBF1 to the transition fiber which functions like a gate of cilia (Yan et al., 2020) (Fig. 1D). Therefore, obesity in Ankrd26-deficient mice might be caused by abnormal cilia gating function and aberrant ciliary import of GPCRs implicated in body weight control. In contrast to ANKRD26, neuron-specific Talpid3 depletion does not cause obesity (Bashford and Subramanian, 2019). Therefore, there might be a differential role of ANKRD26 and TALPID3 in the cilia-related functions in different types of neurons.
CLOSING REMARKS
Accumulated evidence supports that cilia–mediated signaling in specific hypothalamic neuronal populations is critical for the maintenance of energy balance and normal body weight. To date, the proposed role of the primary cilia is to provide a signaling platform for neuropeptides that control the energy balance and feeding behaviors. The current cilia-localized candidate receptors are MC4R, NPY2R, NPY5R, MCHR1, SSTR3, 5HT6, dopamine receptor 1 (D1), and others. The relationship between MC4R and the primary cilia is well-studied in relation to human obesity whereas the physiological and biological roles of other ciliary receptors remain to be elucidated. Moreover, the downstream pathways from cilia signaling that modulate neuronal functions and activity are largely unknown. As adult-induced ablation of cilia in PVH neurons causes slow-onset changes in food intake and body weight (Wang et al., 2021), these effects may require slowly-progressive structural changes in neurons and neuronal circuits. Indeed, in adult-born hippocampal neurons, the cilia modulate glutamatergic synapse formation via the control of dendrite refinement and thereby affect memory formation (Kumamoto et al., 2012). In addition, primary cilia in striatal interneurons regulate synaptic connectivity (Guo et al., 2017). Hence, hypothalamic neuronal cilia may regulate the synaptic integration and neuronal connectivity implicated in weight control. In line with this, the cilia promote axonal projection and dendrite formation in developing POMC neurons by mediating the leptin stimulation of lysosomal proteolysis (Lee et al., 2020).
Although this review article has principally addressed the phenomenon of hypothalamic cilia and obesity, adipocytes transiently express cilia during the early stages of adipogenesis, suggesting that the cilia may have some roles in adipocyte development (Kopinke et al., 2017; Marion et al., 2009; Zhu et al., 2009). Hence, ciliary defects possibly induce obesity through adipocyte mechanisms. In conclusion, the primary cilia in hypothalamic neurons are a tiny but critical organelle for controlling body fatness and defects in these entities can predispose both humans and rodents to obesity.
ACKNOWLEDGMENTS
This study was supported by grants from the National Research Foundation of Korea (NRF), funded by the Ministry of Science and ICT of Korea (2017R1A2B3007123, 2019R1F1A1060805, 2019R1I1A1A01058091, 2020R1A2C 3004843, 2020R1A4A3078962), and from the Asan Institute for Life Sciences (2019-IP0855-1).
Footnotes
AUTHOR CONTRIBUTIONS
C.H.L., G.M.K., and M.S.K. wrote the manuscript.
CONFLICT OF INTEREST
The authors have no potential conflicts of interest to disclose.
REFERENCES
- Acs P., Bauer P.O., Mayer B., Bera T., Macallister R., Mezey E., Pastan I. A novel form of ciliopathy underlies hyperphagia and obesity in Ankrd26 knockout mice. Brain Struct. Funct. 2015;220:1511–1528. doi: 10.1007/s00429-014-0741-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Álvarez-Satta M., Castro-Sánchez S., Valverde D. Alström syndrome: current perspectives. Appl. Clin. Genet. 2015;8:171–179. doi: 10.2147/TACG.S56612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anvarian Z., Mykytyn K., Mukhopadhyay S., Pedersen L.B., Christensen S.T. Cellular signalling by primary cilia in development, organ function and disease. Nat. Rev. Nephrol. 2019;15:199–219. doi: 10.1038/s41581-019-0116-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aznar N., Billaud M. Primary cilia bend LKB1 and mTOR to their will. Dev. Cell. 2010;19:792–794. doi: 10.1016/j.devcel.2010.11.016. [DOI] [PubMed] [Google Scholar]
- Bashford A.L., Subramanian V. Mice with a conditional deletion of Talpid3 (KIAA0586) - a model for Joubert syndrome. J. Pathol. 2019;248:396–408. doi: 10.1002/path.5271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bera T.K., Liu X.F., Yamada M., Gavrilova O., Mezey E., Tessarollo L., Anver M., Hahn Y., Lee B., Pastan I. A model for obesity and gigantism due to disruption of the Ankrd26 gene. Proc. Natl. Acad. Sci. U. S. A. 2008;105:270–275. doi: 10.1073/pnas.0710978105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berbari N.F., Lewis J.S., Bishop G.A., Askwith C.C., Mykytyn K. Bardet-Biedl syndrome proteins are required for the localization of G protein-coupled receptors to primary cilia. Proc. Natl. Acad. Sci. U. S. A. 2008;105:4242–4246. doi: 10.1073/pnas.0711027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berbari N.F., O'Connor A.K., Haycraft C.J., Yoder B.K. The primary cilium as a complex signaling center. Curr. Biol. 2009;19:R526–R535. doi: 10.1016/j.cub.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berbari N.F., Pasek R.C., Malarkey E.B., Yazdi S.M.Z., McNair A.D., Lewis W.R., Nagy T.R., Kesterson R.A., Yoder B.K. Leptin resistance is a secondary consequence of the obesity in ciliopathy mutant mice. Proc. Natl. Acad. Sci. U. S. A. 2013;110:7796–7801. doi: 10.1073/pnas.1210192110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borman A.D., Pearce L.R., Mackay D.S., Nagel-Wolfrum K., Davidson A.E., Henderson R., Garg S., Waseem N.H., Webster A.R., Plagnol V., et al. A homozygous mutation in the TUB gene associated with retinal dystrophy and obesity. Hum. Mutat. 2014;35:289–293. doi: 10.1002/humu.22482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg Y., Overton J., Vaisse C., Leibel R.L., Rost B. In silico mutagenesis: a case study of the melanocortin 4 receptor. FASEB J. 2009;23:3059–3069. doi: 10.1096/fj.08-127530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H., Chen X., Yang Y., Storm D.R. Disruption of type 3 adenylyl cyclase expression in the hypothalamus leads to obesity. Integr. Obes. Diabetes. 2016;2:225–228. doi: 10.15761/IOD.1000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin G.B., Cyr E., Bronson R., Marshall J.D., Gifford E.J., Hicks W., Murray S.A., Zheng Q.Y., Smith R.S., Nishina P.M., et al. Alms1-disrupted mice recapitulate human Alström syndrome. Hum. Mol. Genet. 2005;14:2323–2333. doi: 10.1093/hmg/ddi235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport J.R., Watts A.J., Roper V.C., Croyle M.J., van Groen T., Wyss J.M., Nagy T.R., Kesterson R.A., Yoder B.K. Disruption of intraflagellar transport in adult mice leads to obesity and slow-onset cystic kidney disease. Curr. Biol. 2007;17:1586–1594. doi: 10.1016/j.cub.2007.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C., Li W.D., Geller F., Lei L., Li D., Gorlova O.Y., Hebebrand J., Amos C.I., Nicholls R.D., Price R.A. Possible genomic imprinting of three human obesity-related genetic loci. Am. J. Hum. Genet. 2005;76:427–437. doi: 10.1086/428438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe E., Beales P.L. Bardet-Biedl syndrome. Eur. J. Hum. Genet. 2013;21:8–13. doi: 10.1038/ejhg.2012.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederich R.C., Hamann A., Anderson S., Löllmann B., Lowell B.B., Flier J.S. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat. Med. 1995;1:1311–1314. doi: 10.1038/nm1295-1311. [DOI] [PubMed] [Google Scholar]
- Grarup N., Moltke I., Andersen M.K., Dalby M., Vitting-Seerup K., Kern T., Mahendran Y., Jørsboe E., Larsen C.V.L., Dahl-Petersen I.K., et al. Loss-of-function variants in ADCY3 increase risk of obesity and type 2 diabetes. Nat. Genet. 2018;50:172–174. doi: 10.1038/s41588-017-0022-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D.F., Cui H., Zhang Q., Morgan D.A., Thedens D.R., Nishimura D., Grobe J.L., Sheffield V.C., Rahmouni K. The BBSome controls energy homeostasis by mediating the transport of the leptin receptor to the plasma membrane. PLoS Genet. 2016;12:e1005890. doi: 10.1371/journal.pgen.1005890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D.F., Lin Z., Wu Y., Searby C., Thedens D.R., Richerson G.B., Usachev Y.M., Grobe J.L., Sheffield V.C., Rahmouni K. The BBSome in POMC and AgRP neurons is necessary for body weight regulation and sorting of metabolic receptors. Diabetes. 2019;68:1591–1603. doi: 10.2337/db18-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J., Otis J.M., Higginbotham H., Monckton C., Cheng J., Asokan A., Mykytyn K., Caspary T., Stuber G.D., Anton E.S. Primary cilia signaling shapes the development of interneuronal connectivity. Dev. Cell. 2017;42:286–300.e4. doi: 10.1016/j.devcel.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halaas J.L., Gajiwala K.S., Maffei M., Cohen S.L., Chait B.T., Rabinowitz D., Lallone R.L., Burley S.K., Friedman J.M. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- Han Y.M., Kang G.M., Byun K., Ko H.W., Kim J., Shin M.S., Kim H.K., Gil S.Y., Yu J.H., Lee B., et al. Leptin-promoted cilia assembly is critical for normal energy balance. J. Clin. Invest. 2014;124:2193–2197. doi: 10.1172/JCI69395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W., Ikeda S., Bronson R.T., Yan G., Nishina P.M., North M.A., Naggert J.K. GFP-tagged expression and immunohistochemical studies to determine the subcellular localization of the tubby gene family members. Brain Res. Mol. Brain Res. 2000;81:109–117. doi: 10.1016/S0169-328X(00)00164-9. [DOI] [PubMed] [Google Scholar]
- Hearn T., Spalluto C., Phillips V.J., Renforth G.L., Copin N., Hanley N.A., Wilson D.I. Subcellular localization of ALMS1 supports involvement of centrosome and basal body dysfunction in the pathogenesis of obesity, insulin resistance, and type 2 diabetes. Diabetes. 2005;54:1581–1587. doi: 10.2337/diabetes.54.5.1581. [DOI] [PubMed] [Google Scholar]
- Heydet D., Chen L.X., Larter C.Z., Inglis C., Silverman M.A., Farrell G.C., Leroux M.R. A truncating mutation of Alms1 reduces the number of hypothalamic neuronal cilia in obese mice. Dev. Neurobiol. 2013;73:1–13. doi: 10.1002/dneu.22031. [DOI] [PubMed] [Google Scholar]
- Ishikawa H., Marshall W.F. Ciliogenesis: building the cell's antenna. Nat. Rev. Mol. Cell Biol. 2011;12:222–234. doi: 10.1038/nrm3085. [DOI] [PubMed] [Google Scholar]
- Jacoby M., Cox J.J., Gayral S., Hampshire D.J., Ayub M., Blockmans M., Pernot E., Kisseleva M.V., Compère P., Schiffmann S.N., et al. INPP5E mutations cause primary cilium signaling defects, ciliary instability and ciliopathies in human and mouse. Nat. Genet. 2009;41:1027–1031. doi: 10.1038/ng.427. [DOI] [PubMed] [Google Scholar]
- Kang G.M., Han Y.M., Ko H.W., Kim J., Oh B.C., Kwon I., Kim M.S. Leptin elongates hypothalamic neuronal cilia via transcriptional regulation and actin destabilization. J. Biol. Chem. 2015;290:18146–18155. doi: 10.1074/jbc.M115.639468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S. Adipose tissue malfunction drives metabolic dysfunction in Alström syndrome. Diabetes. 2021;70:323–325. doi: 10.2337/dbi20-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopinke D., Roberson E.C., Reiter J.F. Ciliary Hedgehog signaling restricts injury-induced adipogenesis. Cell. 2017;170:340–351.e12. doi: 10.1016/j.cell.2017.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes M.J., Lowell B.B., Garfield A.S. Melanocortin-4 receptor-regulated energy homeostasis. Nat. Neurosci. 2016;19:206–219. doi: 10.1038/nn.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamoto N., Gu Y., Wang J., Janoschka S., Takemaru K., Levine J., Ge S. A role for primary cilia in glutamatergic synaptic integration of adult-born neurons. Nat. Neurosci. 2012;15:399–405. doi: 10.1038/nn.3042. S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon O., Kim K.W., Kim M.S. Leptin signalling pathways in hypothalamic neurons. Cell. Mol. Life Sci. 2016;73:1457–1477. doi: 10.1007/s00018-016-2133-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechtreck K.F. IFT-cargo interactions and protein transport in cilia. Trends Biochem. Sci. 2015;40:765–778. doi: 10.1016/j.tibs.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.H., Song D.K., Park C.B., Choi J., Kang G.M., Shin S.H., Kwon I., Park S., Kim S., Kim J.Y., et al. Primary cilia mediate early life programming of adiposity through lysosomal regulation in the developing mouse hypothalamus. Nat. Commun. 2020;11:5772. doi: 10.1038/s41467-020-19638-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P., Lechtreck K.F. The Bardet-Biedl syndrome protein complex is an adapter expanding the cargo range of intraflagellar transport trains for ciliary export. Proc. Natl. Acad. Sci. U. S. A. 2018;115:E934–E943. doi: 10.1073/pnas.1713226115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loktev A.V., Jackson P.K. Neuropeptide Y family receptors traffic via the Bardet-Biedl syndrome pathway to signal in neuronal primary cilia. Cell Rep. 2013;5:1316–1329. doi: 10.1016/j.celrep.2013.11.011. [DOI] [PubMed] [Google Scholar]
- Loos R.J., Yeo G.S. The bigger picture of FTO: the first GWAS-identified obesity gene. Nat. Rev. Endocrinol. 2014;10:51–61. doi: 10.1038/nrendo.2013.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubrano-Berthelier C., Dubern B., Lacorte J.M., Picard F., Shapiro A., Zhang S., Bertrais S., Hercberg S., Basdevant A., Clement K., et al. Melanocortin 4 receptor mutations in a large cohort of severely obese adults: prevalence, functional classification, genotype-phenotype relationship, and lack of association with binge eating. J. Clin. Endocrinol. Metab. 2006;91:1811–1818. doi: 10.1210/jc.2005-1411. [DOI] [PubMed] [Google Scholar]
- Marion V., Stoetzel C., Schlicht D., Messaddeq N., Koch M., Flori E., Danse J.M., Mandel J.L., Dollfus H. Transient ciliogenesis involving Bardet-Biedl syndrome proteins is a fundamental characteristic of adipogenic differentiation. Proc. Natl. Acad. Sci. U. S. A. 2009;106:1820–1825. doi: 10.1073/pnas.0812518106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton G.J., Cummings D.E., Baskin D.G., Barsh G.S., Schwartz M.W. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- Nies V.J.M., Struik D., Wolfs M.G.M., Rensen S.S., Szalowska E., Unmehopa U.A., Fluiter K., van der Meer T.P., Hajmousa G., Buurman W.A., et al. TUB gene expression in hypothalamus and adipose tissue and its association with obesity in humans. Int. J. Obes. (Lond.) 2018;42:376–383. doi: 10.1038/ijo.2017.214. [DOI] [PubMed] [Google Scholar]
- Noben-Trauth K., Naggert J.K., North M.A., Nishina P.M. A candidate gene for the mouse mutation tubby. Nature. 1996;380:534–538. doi: 10.1038/380534a0. [DOI] [PubMed] [Google Scholar]
- Ollmann M.M., Wilson B.D., Yang Y.K., Kerns J.A., Chen Y., Gantz I., Barsh G.S. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278:135–138. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- Pampliega O., Orhon I., Patel B., Sridhar S., Díaz-Carretero A., Beau I., Codogno P., Satir B.H., Satir P., Cuervo A.M. Functional interaction between autophagy and ciliogenesis. Nature. 2013;502:194–200. doi: 10.1038/nature12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman J.L., Wheeler M.C., Lloyd D.J., Walker J.R., Glynne R.J., Gekakis N. A gain-of-function mutation in adenylate cyclase 3 protects mice from diet-induced obesity. PLoS One. 2014;9:e110226. doi: 10.1371/journal.pone.0110226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomeroy J., Krentz A.D., Richardson J.G., Berg R.L., VanWormer J.J., Haws R.M. Bardet-Biedl syndrome: Weight patterns and genetics in a rare obesity syndrome. Pediatr. Obes. 2021;16:e12703. doi: 10.1111/ijpo.12703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu L., LeBel R.P., Storm D.R., Chen X. Type 3 adenylyl cyclase: a key enzyme mediating the cAMP signaling in neuronal cilia. Int. J. Physiol. Pathophysiol. Pharmacol. 2016;8:95–108. [PMC free article] [PubMed] [Google Scholar]
- Qu D., Ludwig D.S., Gammeltoft S., Piper M., Pelleymounter M.A., Cullen M.J., Mathes W.F., Przypek R., Kanarek R., Maratos-Flier E. A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature. 1996;380:243–247. doi: 10.1038/380243a0. [DOI] [PubMed] [Google Scholar]
- Rahmouni K., Fath M.A., Seo S., Thedens D.R., Berry C.J., Weiss R., Nishimura D.Y., Sheffield V.C. Leptin resistance contributes to obesity and hypertension in mouse models of Bardet-Biedl syndrome. J. Clin. Invest. 2008;118:1458–1467. doi: 10.1172/JCI32357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh E., Song D.K., Kim M.S. Emerging role of the brain in the homeostatic regulation of energy and glucose metabolism. Exp. Mol. Med. 2016;48:e216. doi: 10.1038/emm.2016.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum J.L., Witman G.B. Intraflagellar transport. Nat. Rev. Mol. Cell Biol. 2002;3:813–825. doi: 10.1038/nrm952. [DOI] [PubMed] [Google Scholar]
- Saeed S., Bonnefond A., Tamanini F., Mirza M.U., Manzoor J., Janjua Q.M., Din S.M., Gaitan J., Milochau A., Durand E., et al. Loss-of-function mutations in ADCY3 cause monogenic severe obesity. Nat. Genet. 2018;50:175–179. doi: 10.1038/s41588-017-0023-6. [DOI] [PubMed] [Google Scholar]
- Sánchez I., Dynlacht B.D. Cilium assembly and disassembly. Nat. Cell Biol. 2016;18:711–717. doi: 10.1038/ncb3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schou K.B., Pedersen L.B., Christensen S.T. Ins and outs of GPCR signaling in primary cilia. EMBO Rep. 2015;16:1099–1113. doi: 10.15252/embr.201540530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M.W., Woods S.C., Porte D., Jr., Seeley R.J., Jr., Baskin D.G., Jr. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- Seo S., Guo D.F., Bugge K., Morgan D.A., Rahmouni K., Sheffield V.C. Requirement of Bardet-Biedl syndrome proteins for leptin receptor signaling. Hum. Mol. Genet. 2009;18:1323–1331. doi: 10.1093/hmg/ddp031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalata A., Ramirez M.C., Desnick R.J., Priedigkeit N., Buettner C., Lindtner C., Mahroum M., Abdul-Ghani M., Dong F., Arar N., et al. Morbid obesity resulting from inactivation of the ciliary protein CEP19 in humans and mice. Am. J. Hum. Genet. 2013;93:1061–1071. doi: 10.1016/j.ajhg.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada M., Tritos N.A., Lowell B.B., Flier J.S., Maratos-Flier E. Mice lacking melanin-concentrating hormone are hypophagic and lean. Nature. 1998;396:670–674. doi: 10.1038/25341. [DOI] [PubMed] [Google Scholar]
- Siljee J.E., Wang Y., Bernard A.A., Ersoy B.A., Zhang S., Marley A., Von Zastrow M., Reiter J.F., Vaisse C. Subcellular localization of MC4R with ADCY3 at neuronal primary cilia underlies a common pathway for genetic predisposition to obesity. Nat. Genet. 2018;50:180–185. doi: 10.1038/s41588-017-0020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singla V., Reiter J.F. The primary cilium as the cell's antenna: signaling at a sensory organelle. Science. 2006;313:629–633. doi: 10.1126/science.1124534. [DOI] [PubMed] [Google Scholar]
- Song D.K., Choi J.H., Kim M.S. Primary cilia as a signaling platform for control of energy metabolism. Diabetes Metab. J. 2018;42:117–127. doi: 10.4093/dmj.2018.42.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stergiakouli E., Gaillard R., Tavaré J.M., Balthasar N., Loos R.J., Taal H.R., Evans D.M., Rivadeneira F., St Pourcain B., Uitterlinden A.G., et al. Genome-wide association study of height-adjusted BMI in childhood identifies functional variant in ADCY3. Obesity (Silver Spring) 2014;22:2252–2259. doi: 10.1002/oby.20840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratigopoulos G., Burnett L.C., Rausch R., Gill R., Penn D.B., Skowronski A.A., LeDuc C.A., Lanzano A.J., Zhang P., Storm D.R., et al. Hypomorphism of Fto and Rpgrip1l causes obesity in mice. J. Clin. Invest. 2016;126:1897–1910. doi: 10.1172/JCI85526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratigopoulos G., Martin Carli J.F., O'Day D.R., Wang L., Leduc C.A., Lanzano P., Chung W.K., Rosenbaum M., Egli D., Doherty D.A., et al. Hypomorphism for RPGRIP1L, a ciliary gene vicinal to the FTO locus, causes increased adiposity in mice. Cell Metab. 2014;19:767–779. doi: 10.1016/j.cmet.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J.S., Yang D.J., Kinyua A.W., Yoon S.G., Seong J.K., Kim J., Moon S.J., Shin D.M., Choi Y.H., Kim K.W. Ventromedial hypothalamic primary cilia control energy and skeletal homeostasis. J. Clin. Invest. 2021;131:e138107. doi: 10.1172/JCI138107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Haley J., Bulgakov O.V., Cai X., McGinnis J., Li T. Tubby is required for trafficking G protein-coupled receptors to neuronal cilia. Cilia. 2012;1:21. doi: 10.1186/2046-2530-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong T., Shen Y., Lee H.W., Yu R., Park T. Adenylyl cyclase 3 haploinsufficiency confers susceptibility to diet-induced obesity and insulin resistance in mice. Sci. Rep. 2016;6:34179. doi: 10.1038/srep34179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vliet-Ostaptchouk J.V., Onland-Moret N.C., Shiri-Sverdlov R., van Gorp P.J., Custers A., Peeters P.H., Wijmenga C., Hofker M.H., van der Schouw Y.T. Polymorphisms of the TUB gene are associated with body composition and eating behavior in middle-aged women. PLoS One. 2008;3:e1405. doi: 10.1371/journal.pone.0001405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Bernard A., Comblain F., Yue X., Paillart C., Zhang S., Reiter J.F., Vaisse C. Melanocortin 4 receptor signals at the neuronal primary cilium to control food intake and body weight. J. Clin. Invest. 2021;131:e142064. doi: 10.1172/JCI142064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Li V., Chan G.C., Phan T., Nudelman A.S., Xia Z., Storm D.R. Adult type 3 adenylyl cyclase-deficient mice are obese. PLoS One. 2009;4:e6979. doi: 10.1371/journal.pone.0006979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Phan T., Storm D.R. The type 3 adenylyl cyclase is required for novel object learning and extinction of contextual memory: role of cAMP signaling in primary cilia. J. Neurosci. 2011;31:5557–5561. doi: 10.1523/JNEUROSCI.6561-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H., Chen C., Chen H., Hong H., Huang Y., Ling K., Hu J., Wei Q. TALPID3 and ANKRD26 selectively orchestrate FBF1 localization and cilia gating. Nat. Commun. 2020;11:2196. doi: 10.1038/s41467-020-16042-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D., Shi S., Wang H., Liao K. Growth arrest induces primary-cilium formation and sensitizes IGF-1-receptor signaling during differentiation induction of 3T3-L1 preadipocytes. J. Cell Sci. 2009;122:2760–2768. doi: 10.1242/jcs.046276. [DOI] [PubMed] [Google Scholar]