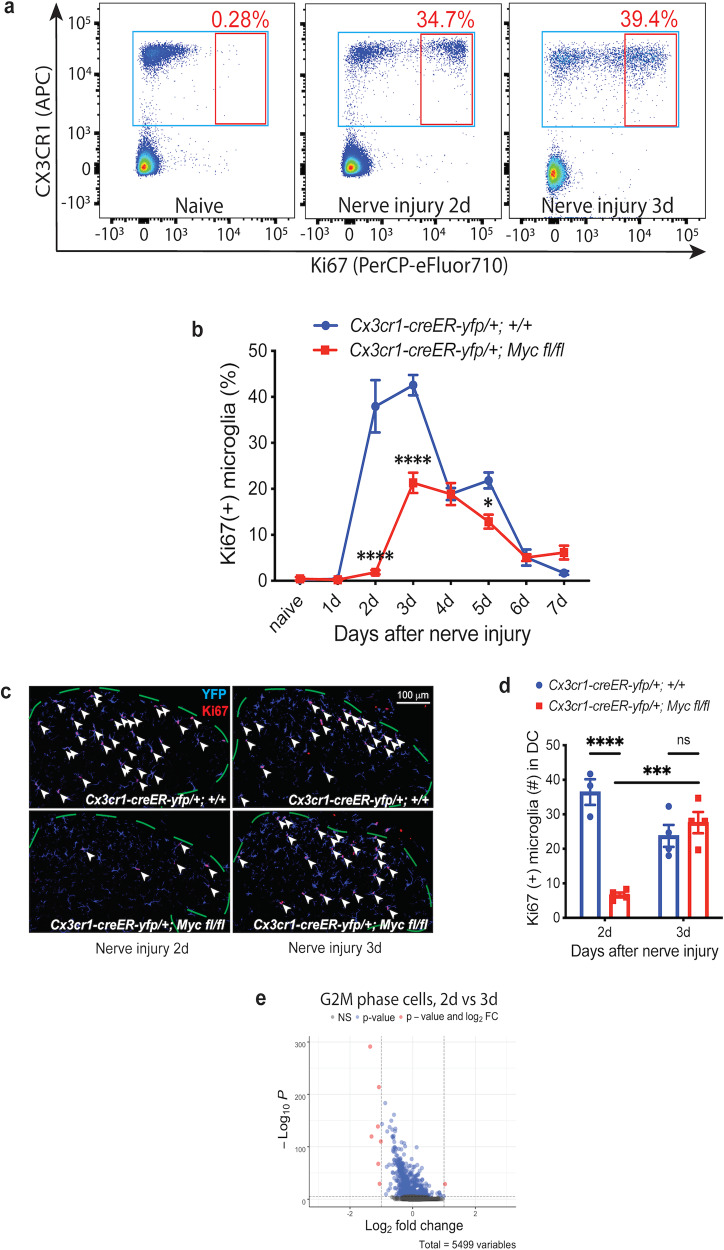
Fig. 2. Microglia proliferation after nerve injury consists of a Myc-dependent early phase and a Myc-independent late phase.
a Representative flow-cytometry scatter plots to assess microglia proliferation in lumbar spinal cord after sciatic nerve injury. The proportion of proliferating Ki67 (+) microglia in Cx3cr1-creER-yfp/+; +/+ animals increased 2 and 3 days after nerve injury. b Quantification of flow cytometry highlights the time course of nerve injury-induced microglia proliferation. In control Cx3cr1-creER-yfp/+; +/+ animals, microglia proliferation started at day 2, persisted through day 3, and then gradually reduced to termination by day 7 after nerve injury. In contrast, the early phase of microglia proliferation on day 2 after nerve injury was completely abolished in Cx3cr1-creER-yfp/+; Myc fl/fl cKO animals, but the late phase and the termination phase of microglia proliferation remained intact in cKO animals. c, d Representative Ki67 IHC staining (c) and the associated quantification (d) of lumbar dorsal cord microglia after nerve injury. Animals with Myc deletion from adult microglia had significantly less Ki67 (+) proliferating microglia in lumbar dorsal cord (DC) 2 days, but not 3 days, after nerve injury. The arrowheads point to the Ki67 (+) microglia, and the dashed lines outline the dorsal lumbar cord. Microglia are identified by YFP staining. e Differential expression volcano plot of pseudobulk scRNA-Seq analysis. The G2/M-phase microglia between day 2 and day 3 after nerve injury displayed distinct gene expression profiles. Analyses are two-way ANOVA with Sidak’s multiple-comparison test, n = 4–6, means ± SEM, *P < 0.05, ***P < 0.001, ****P < 0.0001, ns, not significant. The statistical analysis in b shows the difference between Myc cKO and control animals.