Abstract
Introduction
Edoxaban is the only anti‐Xa inhibitor metabolized in pharmacologically active moiety that could interfere with chromogenic anti‐Xa assays, especially in case of drug‐drug interactions or physiological disorders.
Materials and methods
We evaluated the contribution of the main metabolite of edoxaban, edoxaban‐M4 (M4), in 79 plasma samples from patients taking edoxaban. The total anti‐Xa activity was evaluated on three different chromogenic factor Xa‐based assays. Results were compared with a validated ultra‐high‐performance liquid chromatography coupled with a tandem mass spectrometry measurement. Edoxaban and its active M4 metabolite have also been spiked separately in normal pooled plasma to assess the sensitivity of chromogenic anti‐Xa assays to both molecules individually.
Results
Spiked edoxaban or M4 provided different slopes of linear regression models between chromogenic and chromatographic measurement (from 0.97 for STA Liquid Anti‐Xa to 1.10 for Biophen Heparin LRT Low with edoxaban and from 0.70 for Biophen DiXaI High to 0.83 for Biophen Heparin LRT High, respectively). A positive correlation is observed between the increase of the ratio M4/edoxaban with the difference between chromogenic and chromatographic measurements.
Conclusion
Edoxaban and M4 do not similarly impact chromogenic assays, leading to biased chromogenic estimations of ponderal concentrations. In patient samples, this impact is even more important at low concentrations or in the case of an increase in the M4/edoxaban ratio because of hepatic or renal impairments or in case of drug interactions. This study highlights the limitations and risks of error of expressing results in ponderal concentrations instead of global activity anti‐Xa.
Keywords: anticoagulants, chromatography, drug interactions, edoxaban, high pressure liquid, patients
Essentials.
Edoxaban has active metabolites that may bias chromogenic and chromatographic assays.
UHPLC‐MS/MS and chromogenic methods are compared for the quantification of edoxaban.
Chromogenic assays may overestimate edoxaban concentrations due to active metabolites.
HPLC‐MS/MS do not correlate with the anti‐Xa activity without considering active metabolites.
1. INTRODUCTION
Direct oral anticoagulants (DOACs) include the direct thrombin inhibitor (dabigatran etexilate) and the direct factor Xa (FXa) inhibitors (apixaban, betrixaban, edoxaban, and rivaroxaban). 1 , 2 , 3 , 4 According to the European Society of Cardiology, the American College of Chest Physicians, and the Canadian Cardiovascular Society guidelines, 5 , 6 , 7 , 8 DOACs are preferred over vitamin K antagonists (VKAs) for certain conditions such as nonvalvular atrial fibrillation and venous thromboembolism according to several studies highlighting their favorable benefit‐to‐risk profile. 9 , 10 , 11 , 12 , 13 , 14 However, VKAs are still favorable in patients with mechanical heart valves or for the treatment of triple‐positive antiphospholipid syndrome. 15 , 16 In addition, DOACs present several advantages compared with VKAs, such as (i) no routine laboratory monitoring needed; (ii) smaller inter‐individual variation; (iii) quick onset/offset of action; and (iv) larger target therapeutic range. 4 , 14 , 17 , 18 , 19 Furthermore, they are now widely used in various thromboembolic diseases. Although there is no need of routine monitoring thanks to the predictable pharmacokinetic and pharmacodynamic profiles of DOACs, some clinical situations require to monitor patients while they are on‐treatment (e.g., to assess the degree of anticoagulation before an urgent or elective surgery; before thrombolysis; in case of bleeding episodes or recurrence of thrombotic events; in case of overdosage or suspected progressive accumulation). 2 , 3 , 20 , 21 Edoxaban, which has been available since 2016 under the brand name of Lixiana, received its market authorization for the thromboembolic prevention in patients with nonvalvular atrial fibrillation as well as for the treatment and secondary prevention of deep venous thrombosis and pulmonary embolism. 3 , 22 , 23 As previously reported, among FXa inhibitors, edoxaban is the only compound to release pharmacologically active metabolites (e.g., predominantly edoxaban‐M4 [M4] representing ±10% of total edoxaban and incidentally edoxaban‐M6 [M6], and edoxaban‐M8 [M8], representing less than 10% overall), which could interfere, especially in case of drug‐drug interactions or physiological disorders (i.e., hepatic or renal impairment), with chromogenic anti‐Xa based assays. 19 , 24 Currently, these assays are considered the most appropriate to estimate concentration of FXa inhibitors in clinical practice. 14 However, data are scarce on edoxaban, especially knowing its particular pharmacokinetics and further investigations need to be done. 25 , 26 In the present study, we performed in vitro and ex vivo experiments to evaluate the contribution of the M4 metabolite in plasma sample from patients. We compared the results obtained with different chromogenic assays with those from a validated ultra‐high‐performance liquid chromatography coupled with tandem mass spectrometry (UHPLC‐MS/MS). Although the amount of M4 should be minor in the general population (≤10%), patients with physiological impairment impacting the metabolism or the elimination of edoxaban and its metabolites (e.g., low glomerular filtration rate, liver insufficiency) may present a shift of the parent/metabolite ratio. Also, patients being treated with drugs known to interfere with the pharmacokinetics of edoxaban (e.g., rifampin, ketoconazole, some antiepileptic agents) may present with the same pattern. This switch could interfere with chromogenic anti‐Xa measurements of edoxaban concentrations in plasma and therefore distort edoxaban ponderal concentrations measurements. 4 , 19 , 24
2. MATERIALS AND METHODS
2.1. Testing facilities
The study protocol was in accordance with the Declaration of Helsinki, and the recruitment of the patients was approved by the Ethics Committee of the Cliniques Universitaires Saint‐Luc (Woluwé‐Saint‐Lambert, Brussels, Belgium [CEHF 2017/28DEC/579]). Written informed consent was obtained from each donor. Clinical details are not presented because this is a laboratory investigation study and samples were anonymized. Chromogenic data were generated at QUALIblood s.a. (Namur, Belgium), a contract research organization, whereas UHPLC‐MS/MS measurements were performed at the Department of Pharmacy of the University of Namur (Namur, Belgium).
2.2. Preparation of normal pooled plasma
For the validation of the UHPLC‐MS/MS measurements and spiked plasma experiments, blood was collected and processed from 60 healthy donors. The exclusion criteria and the preparation of normal pooled plasma (NPP) were the same as previously described. 24 , 26 The study protocol was in accordance with the Declaration of Helsinki and the recruitment of the healthy volunteers has been approved by the Ethical Committee of the CHU UCL Namur, Yvoir, Namur, Belgium (49/2009). 24 , 26 Briefly, blood was taken by venipuncture in the antecubital vein and collected into 0.109 M sodium citrate (9:1 v/v) tubes (Venosafe, Terumo, Belgium) using a 21‐gauge needle (Terumo). The platelet‐poor plasma was obtained from the supernatant fraction of blood tubes after a double centrifugation for 15 min at 2500 g at room temperature. Immediately after centrifugations, platelet‐poor plasma from the 60 donors was brought together to obtain the NPP, which was frozen at −80°C without any delay. Frozen NPP samples are thawed at 37°C for 5 min just before the experiment. 26
2.3. Clinical samples
Seventy‐nine plasma samples from patients treated with Lixiana were collected at the Cliniques Universitaires Saint‐Luc (Brussels, Belgium). The inclusion criteria were treatment with edoxaban for at least 2 weeks and obtaining the patients’ informed consent. The exclusion criteria were an age <18 years, an estimated glomerular filtration rate <30 ml/min and geographically inaccessible patients for follow‐up. Plasma samples were collected at Ctrough (i.e., 12 h after the last drug intake) and/or maximum serum concentration (Cmax; i.e., approximately 3 hours after drug intake) according to patient willingness. Blood was taken by venipuncture in the antecubital vein and collected into 0.109 M sodium citrate (9:1 v/v) tubes (SARSTEDT Monovette, Germany) using a 21‐gauge needle. Once collected, the clinical blood samples were processed in the same manner as for the platelet‐poor plasma preparation previously explained (see preparation of the normal polled plasma, discussed previously).
2.4. Edoxaban and edoxaban‐M4 spiked samples
To evaluate the differential impact of edoxaban and its M4 metabolite on chromogenic assays, both moieties were separately spiked in plasma. For this purpose, compounds were separately solubilized in dimethyl sulfoxide at concentrations of 1 mg/ml. Stock solutions were then diluted with phosphate buffered saline. Ten‐fold dilutions were realized to obtain final concentrations ranging from 10 to 500 ng/ml in NPP.
2.5. Liquid chromatography coupled with tandem mass spectrometry
Edoxaban and M4 powder, purchased from Alsachim (Illkirch Graffenstaden, France), were separately solubilized in dimethyl sulfoxide solutions (final concentrations 1 mg/ml), as recommended. Working solutions ranging from 10 to 500 ng/ml of edoxaban and from 10 to 150 ng/ml of M4 were obtained by spiking 10‐fold more concentrated solutions in NPP. In the control experiments, NPP was spiked with phosphate buffered saline solution. 24 Liquid chromatography was performed on an Acquity UHPLC H‐Class system (Waters Corporation, Milford, MA, USA) with an ACQUITY UPLC HSS PFP column (1.7 µm, 2.1 × 100 mm). The liquid chromatography module was coupled with an XEVO TQ‐S triple quadrupole mass spectrometry system (Waters Corporation). The analyses were carried out according to a validated UHPLC‐MS/MS method measurement for edoxaban and M4. 24 Calibration standards, quality controls, and samples from patients were run in triplicate. Concentrations of quality control samples and study samples were calculated in accordance with the calibration curves.
2.6. Chromogenic assays
For plasma samples and spiked samples, concentration of edoxaban was monitored using three calibrated chromogenic anti‐Xa assays. The results were reported as optical density per minute (OD/min) and edoxaban concentration values were obtained by transformation of the OD/min using the calibration curves of each chromogenic method. STA‐Liquid anti‐Xa (Diagnostica Stago, Asnières‐sur‐Seine, France on a STA‐R Max analyzer), Biophen Heparin LRT and Biophen Direct Anti‐Xa Inhibitor (Biophen DiXaI; Hyphen BioMed, Neuville‐sur‐Oise, France on an ACL Top analyzer) were used according manufacturers’ recommendations. For STA Liquid Anti‐Xa, only one calibration curve was performed for the entire range of concentration. For Biophen DiXaI® and Biophen Heparin LRT, two different procedures and calibrations were used (i.e., for the low and the normal ranges using specific sets of edoxaban calibrators).
2.7. Statistical analysis
Calculations and data preprocessing were undertaken with Microsoft Excel 16.47.1 for Mac; statistical analyses and graphics were computed using GraphPad Prism 8.4.3.0D for Mac OS X (GraphPad Software, La Jolla, CA; www.graphpad.com). Descriptive statistics were used when appropriate. To detect assess the linear relationship between chromatographic and chromogenic measurements, Pearson's χ2 test was performed. A p value <0.05 was considered significant. Subsequently, to investigate the distribution density of M4/edoxaban ratios, a one‐sample t‐test was performed. This test also allowed comparison of this ratio distribution with the theoretical ratio of 10%.
To assess the relative difference between the different methodologies for edoxaban quantification, Bland‐Altman and modified Bland‐Altman plots were realized. In the modified Bland‐Altman plots, the x‐axis reports only the values obtained by the UHPLC‐MS/MS method and the y‐axis shows the relative error (%) between the chromogenic methods and the UHPLC‐MS/MS. This error was calculated as follows:
For chromogenic assays, the limits of quantitation (LOQs) were calculated based on manufacturers’ recommendation. For Biophen Heparin LRT (low and normal ranges) as well as for Biophen DiXaI (low and normal ranges), LOQs were calculated based on internal calibration reports:
For STA‐Liquid Anti‐Xa, LOQ was provided by the manufacturer. For all chromogenic assays, the dynamic ranges of quantification were defined as the range between the LOQ and the last calibration point of the calibration. The repeatability of chromogenic assays was defined as the mean of the coefficient of variation ([(standard deviation/mean) ×100]) of the duplicate of each concentration for each test. This repeatability was <5% for each chromogenic test.
3. RESULTS
3.1. Impact of edoxaban and edoxaban‐M4 on chromogenic assays
As observed in Figure 1, spiked samples with edoxaban only showed adequate correlations with the estimated concentrations by chromogenic methods. This was demonstrated by the slopes of the correlation line equations ranging from 0.97 for STA Liquid Anti‐Xa to 1.10 for Biophen Heparin LRT Low. Low methodologies (Biophen DiXaI and Heparin LRT) were unable to estimate concentrations for values higher than or equal to 250 ng/ml of edoxaban. M4 had less impact on FXa‐based chromogenic assays. This was demonstrated by the slopes of the correlation line equations ranging from 0.70 for Biophen DiXaI High to 0.83 for Biophen Heparin LRT High. Low methodologies were unable to estimate concentrations for values greater than or equal to 500 ng/ml of M4.
FIGURE 1.
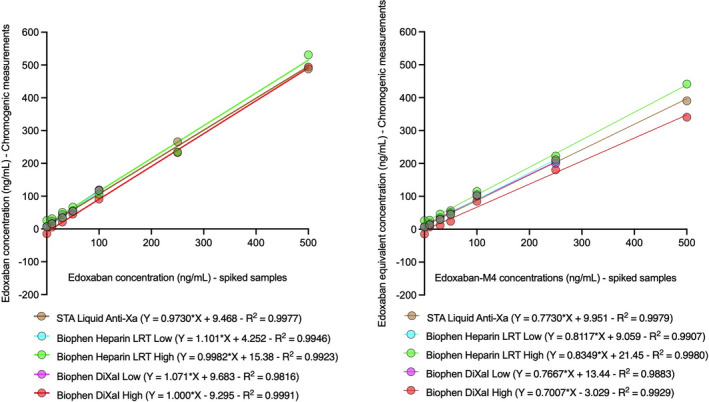
Impact of edoxaban and edoxaban‐M4 on factor Xa‐based chromogenic assays
3.2. Correlation between UHPLC‐MS/MS measurement and chromogenic assays
Pearson's correlation test showed significant correlation between chromatographic and chromogenic measurements. These were slightly improved by adding the contribution of the ponderal concentration of M4 to UHPLC‐MS/MS edoxaban measurement in the cases of STA Liquid anti‐Xa (r = 0.9933 vs r = 0.9947), Biophen DiXaI High (r = 0.9846 vs r = 0.9860), and Heparin LRT High (r = 0.9923 vs r = 0.9948). Linearities were lightly depressed with Biophen DiXaI Low (r = 0.9823 vs r = 0.9791) and Heparin LRT Low (r = 0.9861 vs r = 0.9815).
Linear regressions observed in Figure 2 showed an underestimation of edoxaban concentrations at high concentration with chromogenic assays, regardless of the reagent used.
FIGURE 2.
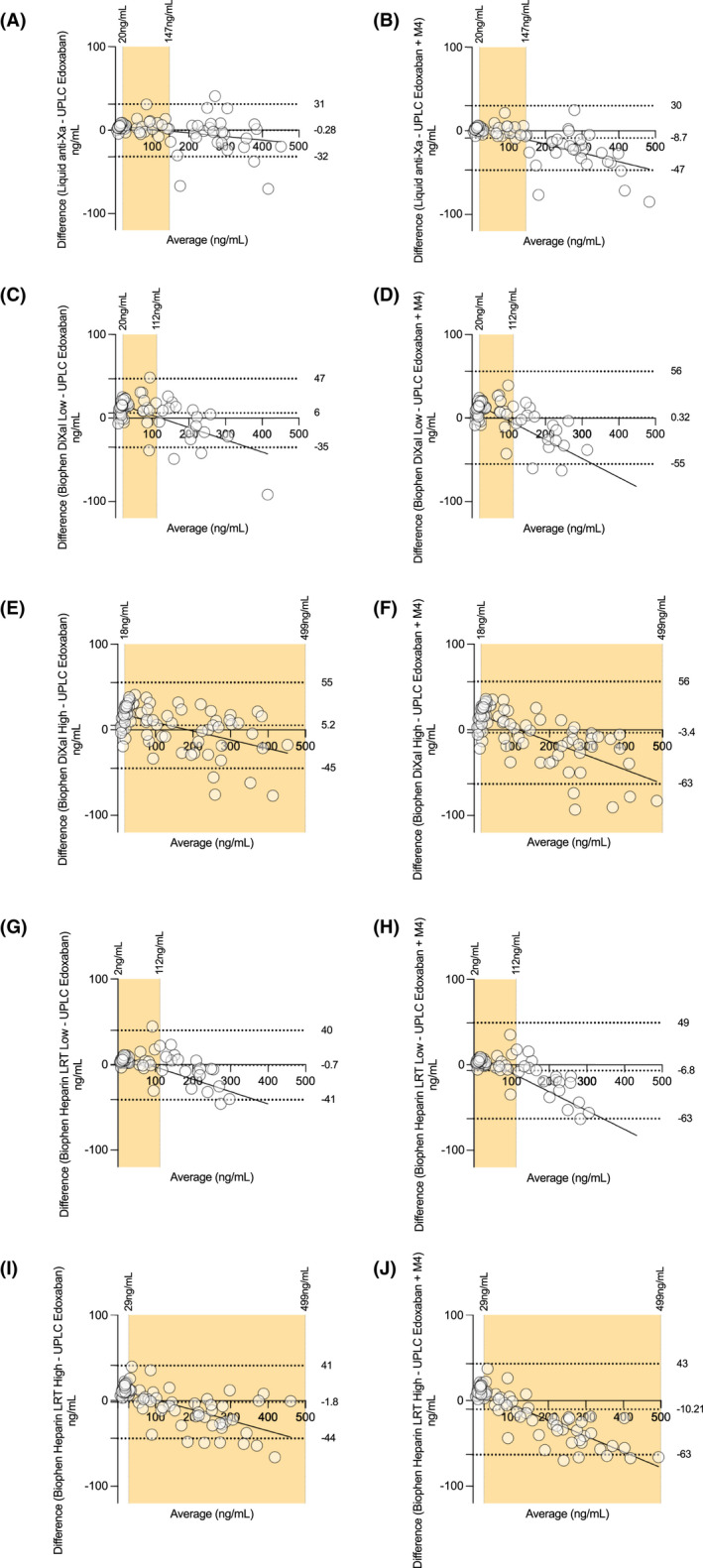
Impact of edoxaban‐M4 on chromogenic assays: Bland‐Altman analysis for chromogenic and ultra‐high‐performance liquid chromatography coupled with tandem mass spectrometry measurements
Figure 3 shows that the relative errors between chromogenic assays and UHPLC measurements tend to fade at high concentrations. Consideration of edoxaban measured alone or with the addition of M4 does not drastically alter these errors.
FIGURE 3.
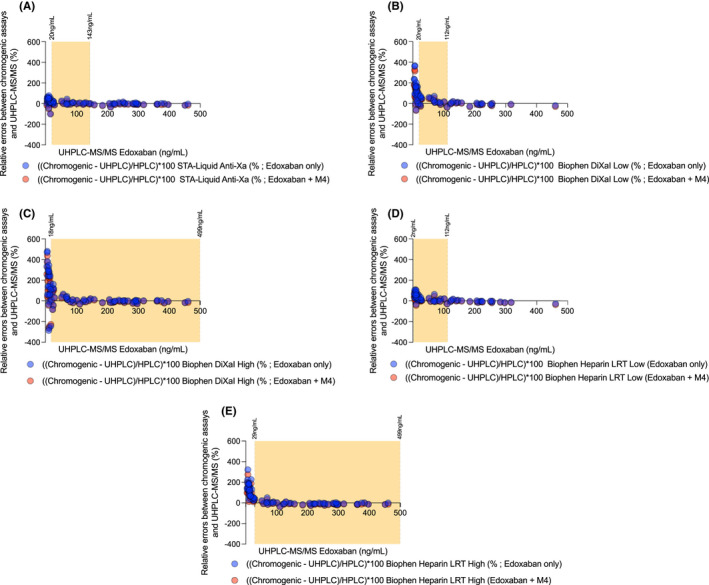
Relative errors between chromogenic assays and ultra‐high‐performance liquid chromatography coupled with tandem mass spectrometry measurements
3.3. Edoxaban‐M4/edoxaban ratios: the impact of edoxaban measurements
One‐sample t‐test of the distribution density of the M4/edoxaban ratios showed a nonsignificantly different dispersion from the theoretical ratio of 10% (p = 0.1204) for the patient samples. Nevertheless, one‐sample t‐test showed a significant difference between samples collected at Ctrough (p = 0.02760) and those collected at Cmax (p ≤ 0.0001) (Figure 4). As shown in Figure 5 and in Table S1, we observed an increasing trend between the M4/edoxaban ratio and the difference measured between the chromogenic and chromatographic method.
FIGURE 4.
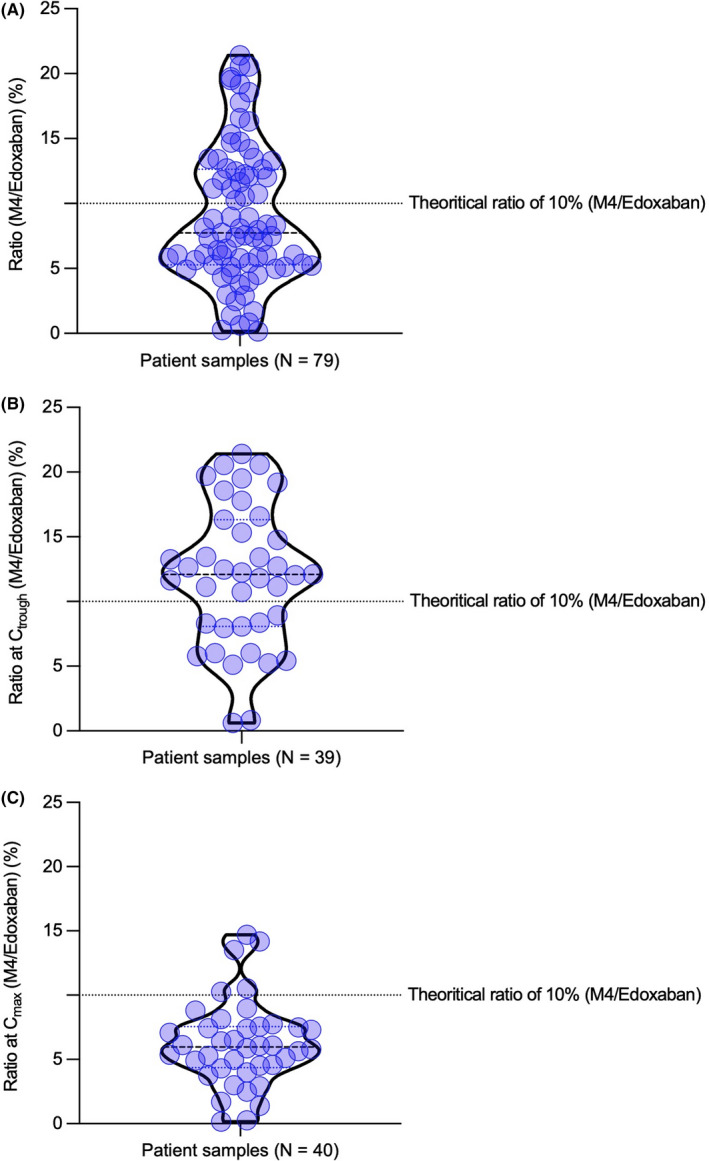
Density distribution of edoxaban‐M4/edoxaban ratios for patients
FIGURE 5.
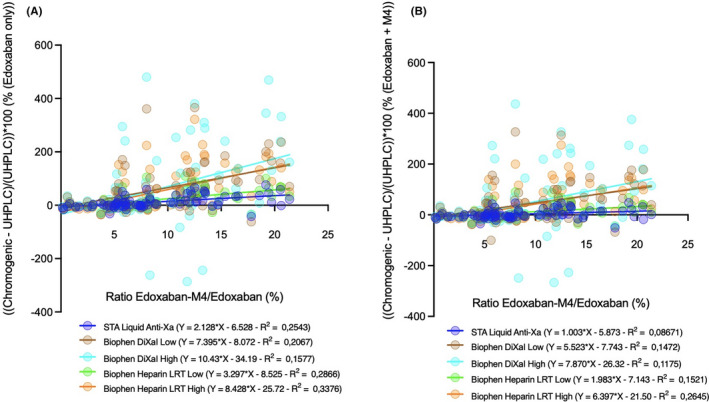
Relationship between edoxaban‐M4/edoxaban ratio and difference (%) between chromogenic and chromatographic methods
4. DISCUSSION
Although routine monitoring is generally not required for the direct FXa (i.e., apixaban, rivaroxaban, and edoxaban) and FIIa (dabigatran) inhibitors because of predictable pharmacokinetic and pharmacodynamic profiles, it should be considered for edoxaban, owing to physiologically active metabolites (M4, M6, and M8), especially in the case of drug‐drug interactions or physiological impairment such as renal or hepatic insufficiency. In this study, we aimed to evaluate the impact of edoxaban and its active metabolite, M4, on three common chromogenic FXa‐based assays in edoxaban‐treated patients. The measurements were compared with a gold‐standard, validated UHPLC‐MS/MS method for both parent and metabolite quantification in plasma.
4.1. Edoxaban and edoxaban‐M4: A different impact on chromogenic assays
As observed, the impact of the same amount of edoxaban or M4 on chromogenic assays is significantly different. On the one hand, edoxaban only provides a good correlation between chromatographic and chromogenic assays. For a given M4 concentration, the ponderal concentration of edoxaban will be underestimated by chromogenic assays. This difference will be even more pronounced in a patient with drug interactions or liver or kidney impairment. Therefore, because of a different impact, it is foreseeable that the presence of M4 tends to lower the edoxaban concentration provided by a chromogenic measurement. As reported in Figure 2, Bland‐Altman plots showed that chromogenic assays calibrated with edoxaban underestimate edoxaban concentrations when compared with UHPLC‐MS/MS measurements. Linear regressions showed a tendency to underestimate edoxaban concentrations, especially at high concentrations (Figure 2). Low methodologies (Biophen DiXaI and Heparin LRT) were unable to estimate concentrations of edoxaban or M4 for values higher than or equal to 250 or 500 ng/ml, respectively, because these concentrations fall outside the manufacturer's validated measurement range.
4.2. Different pharmacokinetics: What are the consequences?
As already mentioned, the pharmacokinetic profiles of edoxaban and M4 are different. Indeed, according to Bathala et al., 27 edoxaban and M4 show a Cmax of 332 and 22 ng/ml, Tmax of 0.5 and 1.8 h and T1/2 of 7.4 and 8.2 h, respectively. Therefore, the time at which the sampling is performed can have an impact on chromogenic and chromatographic results. 27 This Cmax lag time between the parent compound and the metabolite may result in a biased and falsely diminished assessment between the chromogenic and chromatographic methods, as mentioned previously. To minimize bias in the results, sampling should be performed at the Cmax of edoxaban (0.5 h) and not the Cmax of M4.
By doing so, the impact of M4 would be limited and the measured concentrations would be more accurately assessed by a chromogenic assay than if the sampling had been performed after 1.8 h, at the Cmax of M4.
4.3. Drug interactions and physiological impairment: A non‐negligible impact
As already mentioned, both drug interactions (i.e., potent P‐gp or cytochromes inhibitors or inducers) and physiological impairments, such as liver and renal deficiency, can lead to significantly increased M4 concentrations. Indeed, in mild (creatinine clearance [CrCl] ≥50‐≤80 ml/min), moderate (CrCl ≥30‐<50 ml/min), and severe (CrCl <30 ml/min) renal impairment, the exposure of patients to edoxaban‐M4 is 2.25, 3.74, and 3.91 times higher than in a patient with normal CrCl. This increase will be even greater if there are extrinsic factors influencing the pharmacokinetics of edoxaban such as the presence of drug interactions. For example, concomitant use of ketoconazole (a P‐gp transporter and CYP3A4 inhibitor) increases total edoxaban exposure by 87%, whereas intakes of cyclosporine or erythromycin lead to increased exposures by 73% and 85%, respectively. 24 , 28 , 29 , 30 , 31 , 32 , 33 This significant increase in the metabolite, given its different impact from edoxaban on chromogenic assays, may bias the measurement by decreasing the “equivalent edoxaban” concentration. Although drug interactions should be avoided whenever possible for obvious reasons, the concentrations of both edoxaban and its metabolites should be assessed in such situations. Chromogenic tests are not able to distinguish the parent compound from the active metabolites, and for that reason chromatographic method should be advocated for clinical decision making in such situations.
4.4. Ponderal concentration or anti‐Xa activity: A point of debate
Finally, the most important issue raised by the present study is the interpretation of the results provided by the chromatographic or chromogenic measurement. The influence of the patient's general condition (mild to severe renal or hepatic insufficiency) or the presence of drug interactions are not problematic for a chromatographic measurement considering the parent compound and its metabolites. The interest of such a measurement will allow the concomitant evaluation of the components and will make it possible to detect their accumulation. Conversely, a chromogenic measurement calibrated with edoxaban may be biased by the increased amount of M4. Chromogenic assays are not similarly impacted by edoxaban and its metabolite M4 and therefore the interpretation of the result obtained may be distorted. This raises the question about the need to report a result in overall anti‐Xa activity rather than ponderal concentrations; although both results will be impacted by metabolites, the former will better reflect the patient's anticoagulation status. This perspective has already been proposed by Joost Van Pelt et al with the DaXa‐inhibition assay 34 and would be even more interesting in the case of molecules with active metabolites. For this, further studies are needed to reevaluate the different decision cutoffs for the different DOACs.
For this study, some limitations must be disclosed. First, we were only able to consider samples of patients with no drug interactions with edoxaban. A follow‐up investigation will be required to complete this pilot study by including patients with potential drug interactions or physiological failure that may cause an accumulation of the active metabolite. 26 , 35 , 36 , 37 , 38 , 39 , 40 In addition, our study only considers the M4 without considering M6 and M8. Nevertheless, as previously mentioned, IC50 and Cmax of M6 (6.9 nM and 8.55 ng/ml) and M8 (2.7 nM and 0.63 ng/ml) should be negligible contrary to the impact of the M4 (1.8 nM and 23.1 ng/ml) with parent edoxaban (3.0 nM and 243 ng/ml) in absence of CYP inducers. 24 Furthermore, no manufacturer has been able to offer us the M6 and M8 in sufficient quantity and with acceptable purity for UHPLC‐MS/MS. In addition, we were unable to obtain information on the race/ethnicity of the individuals who participated in the study, which may play a possible role with regard to inter‐individual variability and in particular on the coagulation of patients. This represents a limitation in terms of pharmacogenetics where the notion of ethnicity cannot be considered for inter‐individual comparison.
Finally, the impact of mild, moderate, or severe renal and hepatic impairment on the M4/edoxaban ratio and therefore on edoxaban monitoring remains to be assessed. Although a theoretical impact can be expected, some studies seem to point to a negligible impact on monitoring. 41 , 42 However, these studies have only assessed the impact on chronometric measurements on small cohorts which have already demonstrated limitations in monitoring from inter‐individual and inter‐reagent variability.
5. CONCLUSION AND PERSPECTIVES
The era of direct oral anticoagulants is still far from over. Through years of development and continuous evaluation of these medications, numerous studies have allowed us to assess the limitations of analytical methods. We have reached a stage where the expression of chromogenic results in the units of weight shows limitations that need to be taken into consideration. This study highlights some limitations of both gold‐standard UHPLC‐MS/MS and chromogenic assays for the quantification of DOAC concentrations. On the one hand, without consideration of physiologically active metabolites, a chromatographic measurement of the parent compound solely is not representative of the real anti‐Xa activity. In addition, the high turnaround time and the time between sampling and results makes the use of UHPLC‐MS/MS in clinical routine challenging. Nevertheless, the chromatographic method allows, considering a concomitant measurement of active metabolites, to detect accumulations of active compounds, parent or metabolites, which is interesting in case of drug interactions of hepatic or renal impairments. On the other hand, chromogenic tests, although faster, do not allow to distinguish the parent from the active metabolites. Because the metabolites and the parent compound do not have an equivalent impact on the test, an expression of the result in weight concentration of edoxaban is not justified. To avoid such limitations, calibrations involving concomitantly edoxaban and its active metabolites should be preferred to evaluate the global anti‐Xa activity. This will avoid, especially in case of drug‐drug interactions (e.g., P450 cytochrome or transporters inducers) or physiological disorders (renal or hepatic insufficiency), a risk of inadequate control of coagulation from different contribution of the metabolites to the total anti‐Xa activity, which serves for the estimation of edoxaban concentration. It would be wise to reevaluate the interpretation of the absorbance measurements on which the chromogenic tests are based to be able to neglect the presence of possible active metabolites. This is even more important at low concentrations. Indeed, the decision cutoffs for surgeries with bleeding risks are expressed in ng/ml. Underestimation of edoxaban concentrations by chromogenic measurement could lead to misinterpretation. A potential improvement to avoid these errors would be to modify the expression of the results. Like the international normalized ratio for the monitoring of AVK, a chromogenic ratio of an absorbance measurement in OD/min compared with a reference would allow an evaluation of the patient's coagulation status, making the decision‐making safe. Additional analyses are therefore necessary to complete this pilot study with a large cohort of patients experiencing drug interactions with edoxaban. These limitations highlighted in this study with edoxaban should also be assessed for FIIa inhibitors (currently only dabigatran is marketed). 43
RELATIONSHIP DISCLOSURE
J. Douxfils is chief executive officer and founder of Qualiblood s.a. and reports personal fees from Mithra Pharmaceuticals, Gedeon Richter, Norgine, Portola, YHLO, Werfen, Stago, Roche, Roche Diagnostics, and Daiichi‐Sankyo, outside the submitted work. François Mullier reports institutional fees from Stago, Werfen, Nodia, Sysmex, and Bayer; he also reports speaker fees from Boehringer Ingelheim, Bayer Healthcare, and Bristol‐Myers Squibb‐Pfizer, all outside the submitted work. Hélène Haguet is assay director at Qualiblood s.a. Celine Bouvy is an employee of Qualiblood s.a. The other authors have no conflicts of interest to disclose.
AUTHOR CONTRIBUTIONS
Romain Siriez wrote the different drafts and final manuscript and was responsible for the data curation and visualization. Philippe Hainaut and Halil Yildiz provided resources of plasma samples from patients treated with edoxaban. Vincent Maloteau was responsible of the UHPLC‐MS/MS investigation and data analysis under the supervision of Romain Siriez. Céline Bouvy supervised chromogenic assays at QUALIblood s.a., whereas Hélène Haguet was responsible for the validation of chromogenic data. Reviewing and editing were provided by all authors, which performed critical feedback and helped shape the research, analysis, and formal analysis. Jean‐Michel Dogné was responsible for the conceptualization, methodology, funding acquisition and supervision of the project. All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
Supporting information
Table S1
ACKNOWLEDGMENTS
The authors acknowledge Anne‐Sophie Delvigne; Elise Modafari; Laure Morimont; and Sabrina Melchionda from Qualiblood s.a. for their contribution in this study. The authors also thank their commercial partners, Diagnostica Stago and Hyphen‐BioMed/Nodia, for providing reagents for chromogenic assays.
Siriez R, Yildiz H, Bouvy C, et al. The edoxaban‐M4 metabolite and measurement of edoxaban by chromogenic assays in human plasma. Res Pract Thromb Haemost. 2022;6:e12680. doi: 10.1002/rth2.12680
Handling Editor: Dr Johnny Mahlangu
Funding information
Chromogenic assays were a kind gift from Diagnostica Stago and Hyphen BioMed
REFERENCES
- 1. Yeh CH, Hogg K, Weitz JI. Overview of the new oral anticoagulants: opportunities and challenges. Arterioscler Thromb Vasc Biol. 2015;35(5):1056‐1065. [DOI] [PubMed] [Google Scholar]
- 2. Stacy Z, Richter S. Practical considerations for the use of direct oral anticoagulants in patients with atrial fibrillation. Clin Appl Thromb Hemost. 2017;23(1):5‐19. [DOI] [PubMed] [Google Scholar]
- 3. Stacy ZA, Richter SK. Direct oral anticoagulants for stroke prevention in atrial fibrillation: treatment outcomes and dosing in special populations. Ther Adv Cardiovasc Dis. 2018;12(9):247‐262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moner‐Banet T, Alberio L, Bart PA. Does one dose really fit all? On the monitoring of direct oral anticoagulants: a review of the literature. Hamostaseologie. 2020;40(2):184‐200. [DOI] [PubMed] [Google Scholar]
- 5. Corrigendum to: 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio‐Thoracic Surgery (EACTS). Eur Heart J. 2021;42(5):507. [DOI] [PubMed] [Google Scholar]
- 6. Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio‐Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42(5):373‐498. [DOI] [PubMed] [Google Scholar]
- 7. Lip GYH, Banerjee A, Boriani G, et al. Antithrombotic therapy for atrial fibrillation: CHEST guideline and expert panel report. Chest. 2018;154(5):1121‐1201. [DOI] [PubMed] [Google Scholar]
- 8. Andrade JG, Aguilar M, Atzema C, et al. The 2020 Canadian Cardiovascular Society/Canadian Heart Rhythm Society comprehensive guidelines for the management of atrial fibrillation. Can J Cardiol. 2020;36(12):1847‐1948. [DOI] [PubMed] [Google Scholar]
- 9. Schulman S, Kakkar AK, Goldhaber SZ, et al. Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation. 2014;129(7):764‐772. [DOI] [PubMed] [Google Scholar]
- 10. Investigators E, Bauersachs R, Berkowitz SD, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363(26):2499‐2510. [DOI] [PubMed] [Google Scholar]
- 11. Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369(9):799‐808. [DOI] [PubMed] [Google Scholar]
- 12. Hokusai VTEI, Buller HR, Decousus H, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med. 2013;369(15):1406‐1415. [DOI] [PubMed] [Google Scholar]
- 13. Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361(24):2342‐2352. [DOI] [PubMed] [Google Scholar]
- 14. Douxfils J, Adcock DM, Bates SM, et al. 2021 Update of the international council for standardization in haematology recommendations for laboratory measurement of direct oral anticoagulants. Thromb Haemost. 2021;121(8):1008‐1020. [DOI] [PubMed] [Google Scholar]
- 15. Steffel J, Collins R, Antz M, et al. 2021 European Heart Rhythm Association practical guide on the use of non‐vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Europace. 2021;23(10):1612‐1676. [DOI] [PubMed] [Google Scholar]
- 16. Cerda P, Becattini C, Iriarte A, Hernandez JC, Corbella X, Riera‐Mestre A. Direct oral anticoagulants versus vitamin K antagonists in antiphospholipid syndrome: a meta‐analysis. Eur J Intern Med. 2020;79:43‐50. [DOI] [PubMed] [Google Scholar]
- 17. Testa S, Tripodi A, Legnani C, et al. Plasma levels of direct oral anticoagulants in real life patients with atrial fibrillation: results observed in four anticoagulation clinics. Thromb Res. 2016;137:178‐183. [DOI] [PubMed] [Google Scholar]
- 18. Nehaj F, Sokol J, Ivankova J, Mokan M, Mokan M, Stasko J. Edoxaban affects TRAP‐dependent platelet aggregation. J Thromb Thrombolysis. 2020;49(4):578‐583. [DOI] [PubMed] [Google Scholar]
- 19. Tobe A, Osanai H, Tanaka A, et al. Comparison of anti‐factor Xa activity among three different factor Xa inhibitors in non‐valvular atrial fibrillation patients with renal impairment. Clin Drug Investig. 2020;40(6):567‐573. [DOI] [PubMed] [Google Scholar]
- 20. Gosselin R, Adcock Funk DM, Douxfils J. An update on laboratory assessment for direct oral anticoagulants (DOACs). Int J Lab Hematol. 2019;41(S1):33‐39. [DOI] [PubMed] [Google Scholar]
- 21. Douxfils J, Ageno W, Samama CM, et al. Laboratory testing in patients treated with direct oral anticoagulants: a practical guide for clinicians. J Thromb Haemost. 2018;16(2):209‐219. [DOI] [PubMed] [Google Scholar]
- 22. CBIP Sang et coagulation ‐ Anticoagulants ‐ Anticoagulants oraux directs (OAD) ‐ Edoxaban 2021. https://www.cbip.be/fr/chapters/3?frag=25593 (accessed 13 October 2021).
- 23. Almenglo C, Mosquera‐Garrote N, Gonzalez‐Peteiro M, Gonzalez‐Juanatey JR, Alvarez E. Edoxaban's contribution to key endothelial cell functions. Biochem Pharmacol. 2020;178:114063. [DOI] [PubMed] [Google Scholar]
- 24. Siriez R, Alpan L, Elasaad K, et al. Importance of measuring pharmacologically active metabolites of edoxaban: development and validation of an ultra‐high‐performance liquid chromatography coupled with a tandem mass spectrometry method. J Thromb Thrombolysis. 2020;49(3):395‐403. [DOI] [PubMed] [Google Scholar]
- 25. Hillarp A, Strandberg K, Baghaei F, Fagerberg Blixter I, Gustafsson KM, Lindahl TL. Effects of the oral, direct factor Xa inhibitor edoxaban on routine coagulation assays, lupus anticoagulant and anti‐Xa assays. Scand J Clin Lab Invest. 2018;78:575‐583. [DOI] [PubMed] [Google Scholar]
- 26. Douxfils J, Chatelain B, Chatelain C, Dogne JM, Edoxaban MF. Impact on routine and specific coagulation assays. A practical laboratory guide. Thromb Haemost. 2016;115(2):368‐381. [DOI] [PubMed] [Google Scholar]
- 27. Bathala MS, Masumoto H, Oguma T, He L, Lowrie C, Mendell J. Pharmacokinetics, biotransformation, and mass balance of edoxaban, a selective, direct factor Xa inhibitor, in humans. Drug Metab Dispos. 2012;40(12):2250‐2255. [DOI] [PubMed] [Google Scholar]
- 28. Parasrampuria DA, Truitt KE. Pharmacokinetics and pharmacodynamics of edoxaban, a non‐vitamin K antagonist oral anticoagulant that inhibits clotting factor Xa. Clin Pharmacokinet. 2016;55(6):641‐655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Parasrampuria DA, Mendell J, Shi M, Matsushima N, Zahir H, Truitt K. Edoxaban drug‐drug interactions with ketoconazole, erythromycin, and cyclosporine. Br J Clin Pharmacol. 2016;82(6):1591‐1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. He L, Kochan J, Lin M, Vandell A, Brown K, Depasse F. Determination of edoxaban equivalent concentrations in human plasma by an automated anti‐factor Xa chromogenic assay. Thromb Res. 2017;155:121‐127. [DOI] [PubMed] [Google Scholar]
- 31. Mendell J, Chen S, He L, Desai M, Parasramupria DA. The effect of rifampin on the pharmacokinetics of edoxaban in healthy adults. Clin Drug Investig. 2015;35(7):447‐453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. CBIP . Centre Belge d’Information Pharmacothérapeutique ‐ Bon usage des médicaments ‐ Interactions des médicaments. 2018.
- 33. Vazquez SR. Drug‐drug interactions in an era of multiple anticoagulants: a focus on clinically relevant drug interactions. Hematology. 2018;2018(1):339‐347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. van Pelt LJ, Lukens MV, Testa S, Chatelain B, Douxfils J, Mullier F. The DaXa‐inhibition assay: a concept for a readily available, universal aXa assay that measures the direct inhibitory effect of all anti‐Xa drugs. Thromb Res. 2018;168:63‐66. [DOI] [PubMed] [Google Scholar]
- 35. Douxfils J, Chatelain C, Chatelain B, Dogne JM, Mullier F. Impact of apixaban on routine and specific coagulation assays: a practical laboratory guide. Thromb Haemost. 2013;110(2):283‐294. [DOI] [PubMed] [Google Scholar]
- 36. Douxfils J, Mullier F, Loosen C, Chatelain C, Chatelain B, Dogne JM. Assessment of the impact of rivaroxaban on coagulation assays: laboratory recommendations for the monitoring of rivaroxaban and review of the literature. Thromb Res. 2012;130(6):956‐966. [DOI] [PubMed] [Google Scholar]
- 37. Douxfils J, Mullier F, Robert S, Chatelain C, Chatelain B, Dogne JM. Impact of dabigatran on a large panel of routine or specific coagulation assays. Laboratory recommendations for monitoring of dabigatran etexilate. Thromb Haemost. 2012;107(5):985‐997. [DOI] [PubMed] [Google Scholar]
- 38. Siriez R, Evrard J, Dogne JM, et al. Betrixaban: impact on routine and specific coagulation assays‐a practical laboratory guide. Thromb Haemost. 2018;118(7):1203‐1214. [DOI] [PubMed] [Google Scholar]
- 39. Mani H, Hesse C, Stratmann G, Lindhoff‐Last E. Rivaroxaban differentially influences ex vivo global coagulation assays based on the administration time. Thromb Haemost. 2011;106(1):156‐164. [DOI] [PubMed] [Google Scholar]
- 40. Freyburger G, Macouillard G, Labrouche S, Sztark F. Coagulation parameters in patients receiving dabigatran etexilate or rivaroxaban: two observational studies in patients undergoing total hip or total knee replacement. Thromb Res. 2011;127(5):457‐465. [DOI] [PubMed] [Google Scholar]
- 41. Mendell J, Johnson L, Chen S. An open‐label, phase 1 study to evaluate the effects of hepatic impairment on edoxaban pharmacokinetics and pharmacodynamics. J Clin Pharmacol. 2015;55(12):1395‐1405. [DOI] [PubMed] [Google Scholar]
- 42. Jonsson S, Simonsson US, Miller R, Karlsson MO. Population pharmacokinetics of edoxaban and its main metabolite in a dedicated renal impairment study. J Clin Pharmacol. 2015;55(11):1268‐1279. [DOI] [PubMed] [Google Scholar]
- 43. Meijers JCM, Middeldorp S. An anticoagulant that does not cause bleeding ‐ an abrupt stop on the road to the Holy Grail. J Thromb Haemost. 2019;17(12):2019‐2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


