Figure 1.
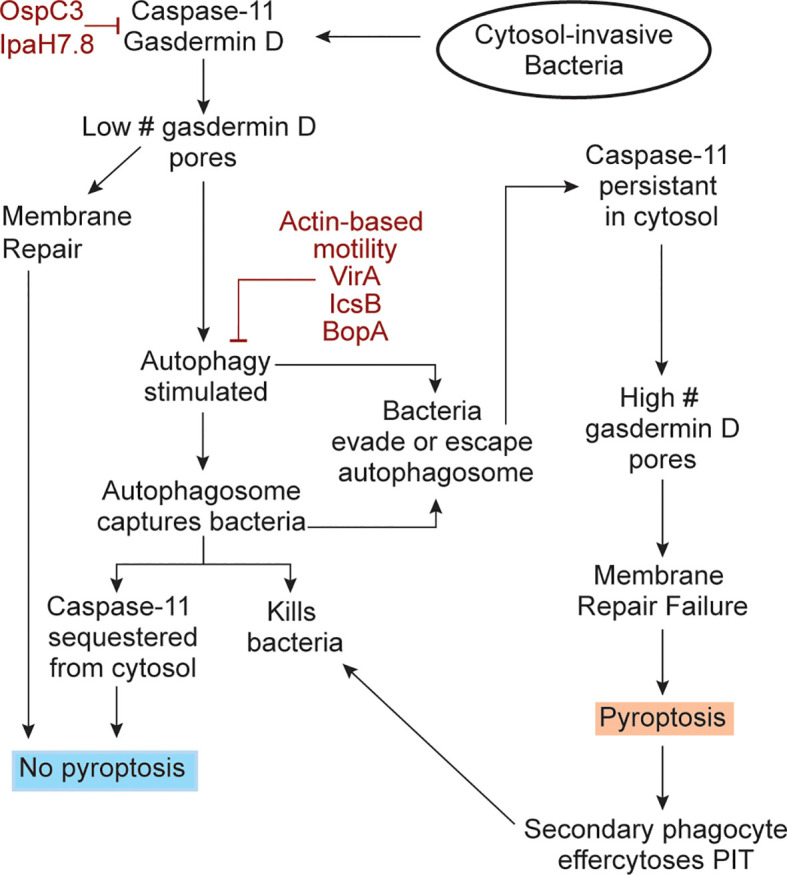
An algorithmic response to cytosol-invasive bacteria. Upon bacterial invasion into the cytosol, caspase-11 is activated by bacterial LPS. Caspase-11 then activates gasdermin D and both simulate the autophagy response. Gasdermin D inserts pores into nearby organelles, such as the mitochondria. The resulting ROS release into the cytosol stimulates autophagy, which captures cytosolic bacteria. There are two significant consequences of this. First, the bacteria can now be killed by the autophagosome. Second, caspase-11 should be simultaneously sequestered from the cytosol and if so, caspase-11 will no longer cleave gasdermin D, thereby preventing pyroptosis. Sequestering of caspase-11 should terminate the gasdermin D cleavage process, and the already generated low amount of gasdermin D pores can be removed from the plasma membrane by membrane repair. If bacterial use virulence factors evade or escape autophagic defenses (burgundy), caspase-11 activity should persist in the cytosol and copious quantities of gasdermin D are expected to be activated. These gasdermin D pores will now insert into the plasma membrane in sufficient quantities and cause pyroptosis. After pyroptosis, the dead cell becomes a pore-induced intracellular trap (PIT) that restrains the bacterium. Secondary phagocytes will be recruited to efferocytose the PITs and the bacteria trapped within it, therefore killing the bacteria. The bacterial virulence factors that inhibit caspase-11, gasdermin D, and the autophagy pathway are shown (burgundy color).
