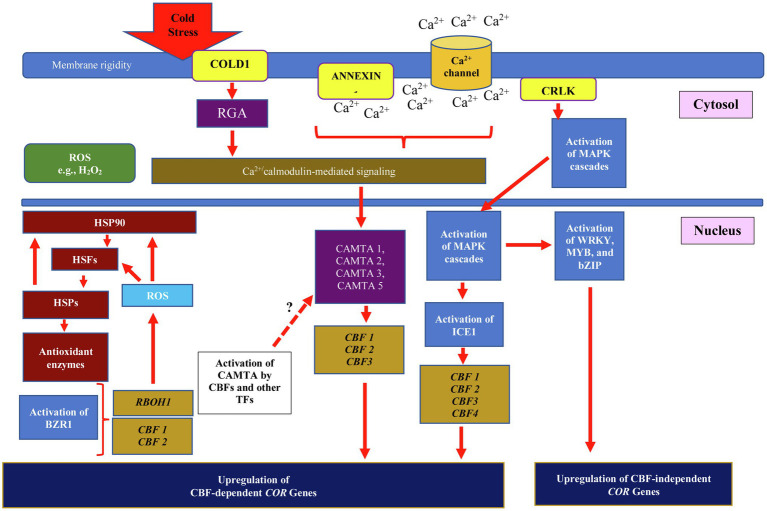
Figure 1.
Integration of cold, heat, and ROS signaling in CBF-dependent and independent pathways for regulating plant cold stress response. Cold stress triggers changes in membrane fluidity and rigidity, and activates the expression of cold-regulated genes (CORs) through CBF-dependent and independent pathways. The chilling tolerance divergence 1 (COLD1) receives external cold stress signal and stimulates rice G-protein A subunit 1 (RGA1) activity. This further activates ANNEXIN1, the Ca2+ channels that transport Ca2+ into the cell. The COLD1/RGA activity and Ca2+ transmit cold stress signal to Ca2+/calmodulin-mediated signaling and Ca2+-dependent protein kinases (CDPKs) located in the cytosol. The CDPKs activities further convey cold stress signal through cytosolic and nuclear MAPKs signaling pathways, which leads to induction of ICE1 activity. ICE1 binds DRE/CRT motif in the promoter of CBF 1, 2, 3, and 4 and upregulates their expression. Integration of cold signal through Ca2+/calmodulin-mediated signaling activates interaction between CAMTAs and CM2 motif located in CBF promoter, which further upregulates COR genes expression. The presence of ABRE, SARE, G-box, W-box, AuXRE, and DRE motifs in CAMTAs promoters suggests the potential regulation of CAMTA by CBFs and other stress-responsive transcription factors (TFs). Accumulation of brassinosteroids promotes binding of BZR1 to E-box found in CBF1 or CBF2 genes and activates the expression of COR genes. Through CBF-independent pathway, the expression of CBF-independent COR genes is regulated through interaction of other TFs such as WRKY, MYB, and bZIP. The expression of CBF-independent COR genes could also be regulated through ROS signaling pathway, involving ROS produced by RESPIRATORY BURST OXIDASE HOMOLOG 1 (RBOH1). The ROS further regulate heat shock factors (HSFs) and heat shock protein 90 (HSP90) and hence develop multi-chaperone network that controls production of antioxidant enzymes. The multi-chaperone network is also involved in controlling HSP90 activity.