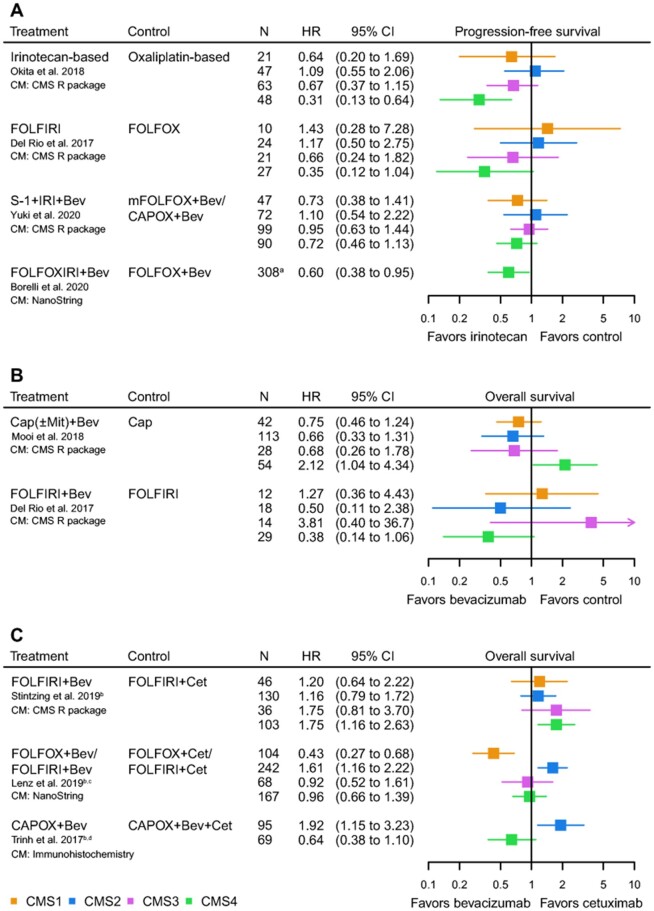
Figure 5.
Forest plots of hazard ratios (HRs) for the predictive value of the consensus molecular subtypes (CMSs) for first-line systemic therapy in metastatic colorectal cancer. A) Progression-free survival for an irinotecan backbone vs control regimen. B) Overall survival for the addition of bevacizumab vs control regimen. C) Overall survival for the addition of bevacizumab vs cetuximab. aTotal number of patients, no information on the number of CMS4 patients. bKRAS wild-type population. c75.2% FOLFOX/24.8% FOLFIRI. dImmunohistochemistry classification: CMS2 and CMS3 depicted as CMS2. Bev = bevacizumab; Cap = capecitabine; CAPOX = capecitabine and oxaliplatin; Cet = cetuximab; CM = classification method; FOLFIRI = 5-fluorouracil, leucovorin, and irinotecan; FOLFOX = 5-fluorouracil, leucovorin, and oxaliplatin; mFOLFOX = modified FOLFOX; FOLFOXIRI, 5-fluorouracil, leucovorin, oxaliplatin and irinotecan; IRI = irinotecan; Mit = mitomycin; N = number of patients.