Summary
Background
In March 2010, Brazil introduced the ten-valent pneumococcal conjugate vaccine (PCV10), which was licensed based on non-inferiority of immunological correlates of protection compared with the seven-valent vaccine. The schedule comprised three primary doses at ages 2 months, 4 months, and 6 months, and a booster dose at age 12 months. A single catch-up dose was offered for children aged 12–23 months at the time of introduction. We assessed PCV10 effectiveness against invasive pneumococcal disease in Brazilian children.
Methods
Invasive pneumococcal disease, defined as isolation of Streptococcus pneumoniae from blood, cerebrospinal fluid, or another normally sterile site, was identified in children age-eligible for at least one PCV10 dose through laboratory-based and hospital-based surveillance in ten states in Brazil from March 1, 2010, until Dec 31, 2012. We aimed to identify four age-matched and neighbourhood-matched controls for each case. We used conditional logistic regression and calculated PCV10 effectiveness as (1–adjusted matched odds ratio) × 100% for vaccine-type and vaccine-related serotypes (ie, in the same serogroup as a vaccine serotype).
Findings
In 316 cases (median age 13·2 months, range 2·6–53·1) and 1219 controls (13·3 months, 2·6–53·1), the adjusted effectiveness of an age-appropriate PCV10 schedule was 83·8% (95% CI 65·9–92·3) against vaccine serotypes, and 77·9% (41·0–91·7) against vaccine-related serotypes. Serotype-specific effectiveness was shown for the two most common vaccine serotypes—14 (87·7%, 60·8–96·1) and 6B (82·8%, 23·8–96·1)—and serotype 19A (82·2%, 10·7–96·4), a serotype related to vaccine serotype 19F. A single catch-up dose in children aged 12–23 months was effective against vaccine-type disease (68·0%, 17·6–87·6). No significant effectiveness was shown against non-vaccine serotypes for age-appropriate or catch-up schedules.
Interpretation
In the routine immunisation programme in Brazil, PCV10 prevents invasive disease caused by vaccine serotypes. PCV10 might provide cross-protection against some vaccine-related serotypes.
Funding
Brazilian Ministry of Health, Pan-American Health Organization, and US Centers for Disease Control and Prevention.
Introduction
Pneumococcal disease is a leading vaccine-preventable cause of childhood mortality worldwide.1 In June, 2009, a ten-valent pneumococcal conjugate vaccine (PCV10), containing ten pneumococcal serotype-specific polysaccharides conjugated to non-typeable Haemophilus influenzae protein D, tetanus toxoid, and diphtheria toxoid (Synflorix, GlaxoSmithKline, Rixensart, Belgium), was licensed in Brazil for routine immunisation of infants. Evidence of PCV10 efficacy against invasive pneumococcal disease was not available at licensure, which was based on immunogenicity data of the comparison of the immune responses to the seven pneumococcal serotypes in a licensed seven-valent pneumococcal conjugate vaccine (PCV7, Prevenar, Pfizer, New York, NY, USA).2
In March, 2010, PCV10 was introduced for routine immunisation of infants through Brazil’s national immunisation programme, which provides publically funded vaccines to all Brazilian children. The schedule comprised three primary doses given to infants at ages 2 months, 4 months, and 6 months and a booster dose at age 12 months. Recommendations for catch-up schedules were three doses by age 11 months plus a booster for children aged 3–7 months at the time of PCV10 introduction, two doses by age 11 months plus booster for those aged 8–9 months, one dose plus booster for those aged 10–11 months, and a single dose for children aged 12–23 months at PCV10 introduction. At the time, no other country had introduced PCV10 nationally, and no post-marketing data for PCV10 effectiveness were available. We assessed PCV10 effectiveness against invasive pneumococcal disease in Brazilian children in a case-control study.
Methods
Setting
Brazil is a high-middle-income Latin American country with about 3 million births per year and a gross domestic product (GDP) per person of US$10 890 (2010).3 Since 1993, Brazil has participated in a Latin American regional laboratory network that does passive surveillance of bacterial pneumonia and meningitis pathogens (SIREVA II),4,5 through which Streptococcus pneumoniae isolates are serotyped and tested for antimicrobial sensitivity. Additionally, Brazil has a well established national meningitis surveillance system and meningitis is a notifiable disease nationwide.6 Pneumococcal isolates from hospitals throughout Brazil are submitted to a national reference laboratory at the Adolfo Lutz Institute, São Paulo, Brazil. Before PCV10 introduction, the Brazilian Ministry of Health invited state health secretariats in ten states (Amazonas, Bahia, Ceara, the Federal District, Goiás, Minas Gerais, Paraná, Pernambuco, Rio Grande do Sul, and São Paulo) to identify hospitals to participate in an enhanced surveillance of invasive pneumococcal disease. A list of participating hospitals is provided in the appendix. The ten states accounted for 66·3% of the population in Brazil in 2009 and 78·4% of the cases of suspected meningitis reported to the national notifiable diseases surveillance system during 2008–09.7
Study population
A case of invasive pneumococcal disease was defined as the detection of S pneumoniae in a normally sterile fluid (eg, blood, cerebrospinal fluid, or pleural fluid) in a child aged 2 months and older who was eligible to have received at least one dose of PCV10. Any cases arising after PCV10 rollout in each of the ten participating states (earliest March 1, 2010, and latest Sept 1, 2010) until Dec 31, 2012, were eligible. Cases were identified though active laboratory-based surveillance at participating hospitals and state reference laboratories. Blood cultures were done at the discretion of the treating physicians. To increase the detection of cases of invasive pneumococcal disease at participating hospitals, the Ministry of Health distributed information about indications for blood culture and provided necessary laboratory supplies.
Cases were initially detected through culture of a normally sterile fluid only. In December, 2010, the protocol was amended to include cases in which S pneumoniae was detected with LytA-targeted real-time PCR8 in cerebrospinal or pleural fluid because laboratories in three states (Bahia, Goiás, and São Paulo) were routinely using this method. Pneumococcal isolates were sent to the Adolfo Lutz Institute for confirmation and serotyping with the Quellung reaction.5 The serotype of cases detected by real-time PCR was ascertained with a multiplex PCR assay for 40 of the most common pneumococcal serotypes.9–11 We classified cases as vaccine-type if the serotype matched any of the serotypes in PCV10 (1, 4, 5, 6B, 7F, 9V, 14, 18C, 19F, and 23F), or as vaccine-related if the serotype was not included in the vaccine but was from the same serogroup as a serotype in the vaccine (6A, 6C, 6D, 7C, 9N, 18A, 18B, 19A, and 23A). Because PCR cannot be used to distinguish between serotypes 6A and 6B, cases with serotype 6A/6B detected with PCR were classified as vaccine-related and were not included in the estimates of serotype-specific effectiveness. All other serotypes were deemed to be non-vaccine types.
For each enrolled case, we aimed to enrol four age-matched and neighbourhood-matched controls. Potential controls were sought through the Information System for Live Births, a national birth registry (with >95% of all births registered)12 that also included all the cases. A list was generated of children born up to 1 month before or after the date of birth of the case and registered in the same neighbourhood in which the case resided at the time of illness. If more than four age-eligible children were identified, the names on the list were randomised and the parents or guardians of the potential controls were approached in that order for enrolment. If fewer than four age-eligible children were identified, potential controls were sought from adjacent neighbourhoods. For potential controls, study staff searched for addresses and phone numbers using public health records (newborn screening records and registration for Brazil’s Family Health Programme) that include this information. To locate potential controls, study staff contacted parents or guardians by telephone; if no telephone number was available, interviewers visited the addresses provided. A minimum of five attempts were made to contact parents or guardians of potential controls; if parents or guardians were not located or declined to participate, the next potential control on the randomly ordered list was contacted. Children residing in the same household as a case and those previously enrolled as a case or control were not eligible to be controls. Like the cases, the controls had to be age-eligible for at least one dose of PCV.
The study protocol was approved by the Brazilian National Committee for Ethical Research and the ethical research committee of the Pan American Health Organization. The US Centers for Disease Control and Prevention deemed the study protocol to be an evaluation of a public health programme and therefore not human subject research. Written informed consent was obtained from all parents or legal guardians of the participating children.
Data gathering
For all participating cases and controls, study personnel did an in-person interview of the parent or guardian of the child using a standardised questionnaire. We gathered demographic and household data including household income, number of people residing in the household, maternal education, and exposure to tobacco smoke in the household. The parent or guardian was asked about chronic diseases in the child, including asplenia, sickle cell disease, haemolytic anaemia, HIV/AIDS, cancer, use of immunosuppressant drugs, organ transplant, diabetes, asthma, and chronic pulmonary, cardiovascular, renal, or hepatic disease. Data were also gathered for day-care attendance, breastfeeding, and other factors potentially associated with both invasive pneumococcal disease and vaccination status. For cases, the medical chart was reviewed to ascertain the presenting clinical syndrome.
Cases and controls were enrolled in the study irrespective of whether vaccine records were available. The primary source of vaccination history data was the child’s immunisation card, obtained from the parent or guardian. If these cards were not available, the vaccination history was sought at the immunisation post where the child was vaccinated. If the parent or guardian reported that the child had never received any vaccines, no written documentation was required and the child was deemed to have received no doses of PCV; all other cases and controls with no documentation of vaccination history were excluded from the analysis of PCV10 effectiveness.
Statistical analysis
Data were double-entered at the Ministry of Health. We defined a reference date for controls as the date on which their age exactly matched the age in days of their corresponding case at the time of hospital admission or medical attention. For analysis, vaccine doses were judged to be valid only if received at least 14 days before hospital admission or medical attention for cases or reference date for controls. Immunisation was classified as up-to-date for PCV10 if the number of valid doses was greater than or equal to the number recommended by age at hospital admission or reference date.
We used conditional logistic regression to calculate the matched odds ratio of PCV10 vaccination versus no vaccination in cases compared with controls. PCV10 effectiveness was calculated with the formula,
We assessed for confounding by including additional variables one by one in the basic models for PCV10 effectiveness. Variables that altered the odds ratio or β for PCV10 effectiveness by at least 20% were included in adjusted multivariable models. We assessed for two-way interactions and correlation between variables included in the adjusted models. Analyses were done with SAS statistical software (version 9.3).
Role of the funding source
The Brazilian Ministry of Health, with support from the Pan American Health Organization and the US Centers for Disease Control and Prevention, funded the surveillance of invasive pneumococcal disease. Health secretariats in participating states provided assistance with data gathering. Participating hospitals were responsible for obtaining pneumococcal isolates. The Pan American Health Organization, through the regional laboratory network project SIREVA II, provided support for the national reference laboratory for invasive pneumococcal diseases. Authors who are employees of the funders and collaborating institutions took full responsibility for the design of the study, data gathering and analysis, and the final decision to publish.
Results
398 cases with laboratory-confirmed invasive pneumococcal disease were identified during the study. Overall, 73 (18%) cases were not included—15 (4%) declined to participate, 26 (7%) could not be located, and 32 (8%) did not have an isolate or had insufficient clinical material available for confirmatory testing. Table 1 shows the characteristics of the 325 cases enrolled in the study; the median age of these cases was 13·3 months (range 2·6–53·1). 49% of 325 cases had meningitis as a clinical syndrome, and 46% of all cases were vaccine-type (table 1). The figure shows that the most common serotype was 14 (73 cases [22%]), followed by serotypes 6B (33 [10%]), 19A (28 [9%]), 3 (28 [9%]), 6A (24 [7%]), and 23F (19 [6%]). No cases were caused by vaccine serotypes 1 or 5, and only one case was due to vaccine serotype 7F. Overall, 77 (24%) cases died, and the most common serotypes in these cases were 14 (17 [22%]), 6A (six [8%]), and 23F (six [8%]).
Table 1:
Characteristics of eligible cases
| Cases (n=325) | |
|---|---|
| Age (months) | |
| Median (range) | 13·3 (2·6–53·1) |
| Clinical syndrome | |
| Meningitis | 158 (49%) |
| Bacteraemic pneumonia | 129* (40%) |
| Pneumonia with effusion | 36 (11%) |
| Bacteraemia† | 2 (<1%) |
| Method of detection | |
| Culture of isolate | 307 (94%) |
| PCR of cerebrospinal fluid or pleural fluid | 18 (6%) |
| Serotype | |
| Vaccine-type‡ | 151 (46%) |
| Vaccine-related§ | 77 (24%) |
| Non-vaccine¶ | 97 (30%) |
| Medical care | |
| Outpatient | 17 (5%) |
| Admitted to hospitalised without intensive care | 187 (58%) |
| Admitted to hospitalised with intensive care | 121 (37%) |
| Deaths (outcome) | |
| Overall | 77 (24%) |
| In cases of meningitis | 57 (36%) |
| In cases of pneumonia | 19 (12%) |
| In cases of bacteraemia | 1 (50%) |
Data are number (%), unless otherwise indicated. PCV10=ten-valent pneumococcal conjugate vaccine.
Includes eight cases with Streptococcus pueumoniae detected in both blood and pleural fluid.
Without pneumonia, meningitis, and sepsis.
PCV10 serotypes 1, 4, 5, 6B, 7F, 9V, 14, 18C, 19F, and 23F.
Serotypes not included in PCV10, but in the same serogroup as any of the included serotypes.
Serotypes not included in PCV10 and not in the same serogroup as any of the included serotypes.
Figure: Pneumococcal serotypes and PCV10 vaccination status in 325 cases of invasive pneumococcal disease.
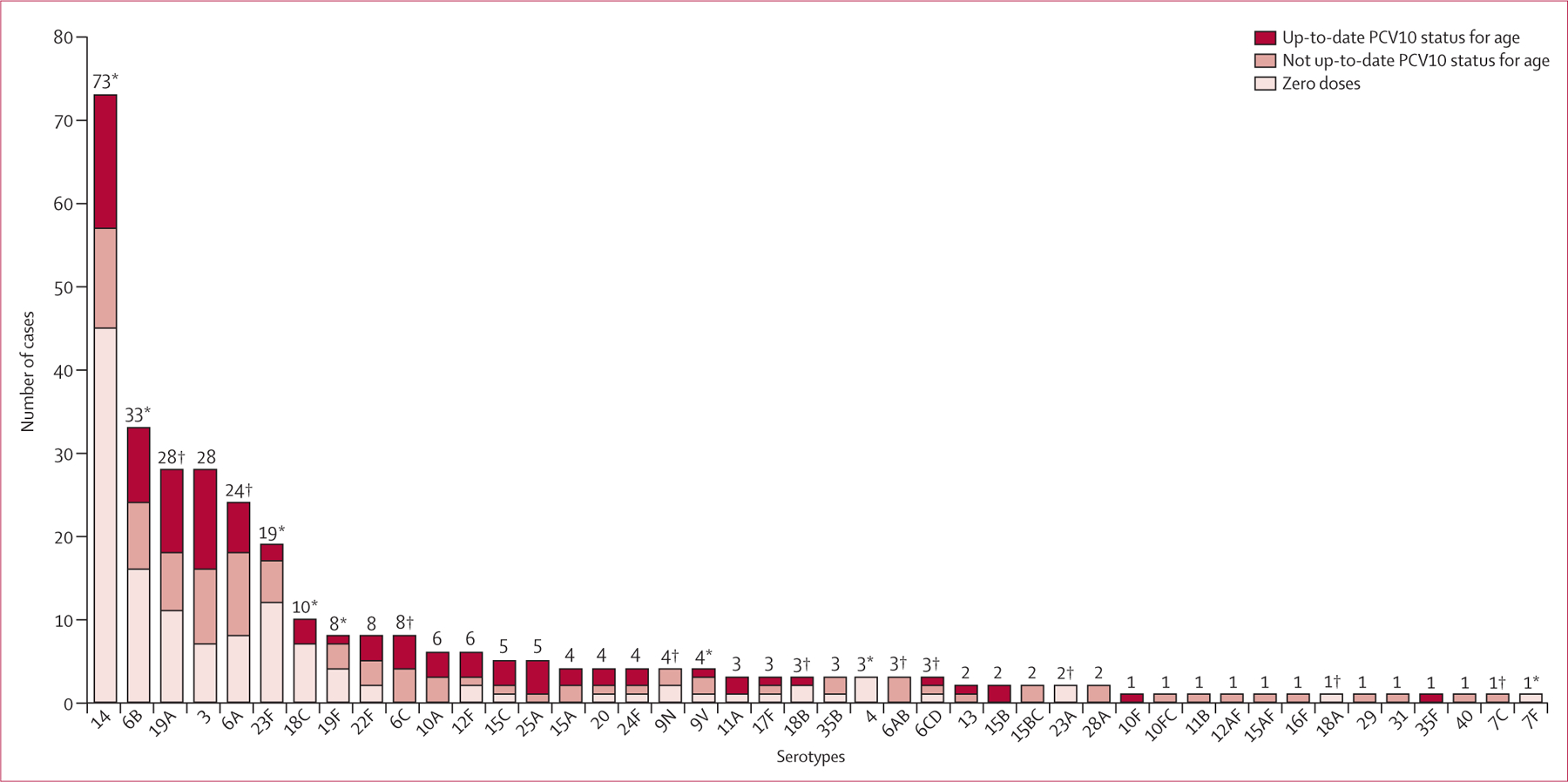
No cases of serotypes 1 or 5 were reported. PCR cannot be used to distinguish between serotypes 6A or 6B, 6C or 6D, 10F or 10C, 12A or 12F, 15B or 15C, or 15A or 15F. Invasive pneumococcal disease cases typed as 6A/6B and 6C/6D were deemed to be vaccine-related serotypes for analyses. PCV10=ten-valent pneumococcal conjugate vaccine. *PCV10 serotypes. †PCV10-related serotypes.
1258 controls (median age 13·7 months [range 2·6–53·1]) were matched to the 325 cases with available serotype. Overall, 1016 (81%) of enrolled controls were from the same neighbourhood as their matched cases, whereas 242 (19%) were from adjacent neighbourhoods. A documented vaccination history was available for all enrolled cases and controls, including five children whose parent or guardian reported that the child had not received any vaccines. Of the 1583 children (325 cases and 1258 controls) enrolled, nine (3%) cases and 39 (3%) controls had received a different pneumococcal vaccine (42 [3%] PCV7, four [<1%] PCV13, and two [<1%] 23-valent polysaccharide), and were therefore excluded from the analysis of PCV10 effectiveness; the remaining children had received PCV10 or had not received any doses of a pneumococcal vaccine. Thus, a total of 316 cases (median age 13·2 months [range 2·6–53·1]) and 1219 matched controls (13·3 months [2·6–53·1]) were included in the effectiveness analysis (table 2).
Table 2:
Characteristics of cases and controls in the PCV10 effectiveness analysis*
| Cases (n=316) | Controls (n=1219) | p value (matched) | |
|---|---|---|---|
| Male sex* | 173 (55%) | 630 (52%) | 0·3181 |
| Maternal education less than 12 years* | 61 (19%) | 255 (21%) | 0·2752 |
| Low household income† | 147 (47%) | 519 (43%) | 0·2098 |
| Crowding‡ | 178 (56%) | 664 (54%) | 0·5973 |
| Any chronic illness*§ | 85 (27%) | 96 (8%) | <0·0001 |
| Asthma | 42 (13%) | 71 (6%) | <0·0001 |
| Premature birth (<37 weeks’ gestation)* | 42 (13%) | 82 (7%) | 0·0004 |
| Low birthweight (<2500 g)* | 39 (12%) | 83 (7%) | 0·0017 |
| Use of immunosuppressant drugs* | 25 (8%) | 22 (2%) | <0·0001 |
| Day care (daily attendance) | 127 (40%) | 357 (29%) | <0·0001 |
| Presence of other children younger than 5 years in the home* | 148 (47%) | 477 (39%) | 0·0110 |
| Exclusive breastfeeding until 3 months of age | 177 (56%) | 804 (66%) | 0·0001 |
| Presence of smoker in the home | 113 (36%) | 387 (32%) | 0·0800 |
| Vaccination history¶ | |||
| At least one dose of diphtheria-tetanus-pertussis-Haemophilus influenzae type B | 295 (93%) | 1196 (98%) | <0·0001 |
| No dose of PCV10 | 129 (41%) | 304 (25%) | Ref |
| One dose of PCV10 | 78 (25%) | 399 (33%) | <0·0001 |
| Two doses of PCV10 | 34 (11%) | 173 (14%) | <0·0001 |
| Three doses of PCV10 | 48 (15%) | 221 (18%) | <0·0001 |
| Four doses of PCV10 | 27 (9%) | 122 (10%) | <0·0001 |
Data are number (%), unless otherwise indicated. PCV10=ten-valent pneumococcal conjugate vaccine. Ref=reference.
Missing data were excluded from denominator.
Defined as monthly household income per household member of less than or equal to 50% of the standard monthly minimum wage.
Defined as a ratio of the number of household members to number of bedrooms greater than two.
Defined as having one or more of the following: asplenia, sickle cell disease, haemolytic anaemia, HIV/AIDS, cancer, use of immunosuppressant drugs, organ transplant, diabetes, asthma, or chronic pulmonary, cardiovascular, renal, or hepatic disease.
Includes only vaccine doses received at least 14 days before reference date, which for cases was the date of hospital admission (or medical attention if not admitted to hospital); for controls, the reference date was that on which their age in days was the same as their corresponding case’s age at hospital admission or medical attention.
Cases and controls included in this analysis were similar in terms of maternal education, income, and crowding (table 2). Chronic illnesses, premature birth, low birthweight, use of immunosuppressant drugs, and the presence of other children younger than 5 years and attendance at daycare were significantly more prevalent in the cases, and exclusive breastfeeding was significantly less prevalent in the cases (table 2). Most children had received at least one dose of diphtheria-tetanus-pertussis-Haemophilus influenzae type B vaccine; however, a significant difference in coverage with this vaccine was noted between cases (93%) and controls (98%; p<0·0001; table 2). Overall, 187 (59%) cases had received at least one dose of PCV10, including 61 (41%) of 147 with vaccine-type disease, 48 (64%) of 75 with vaccine-related disease, and 78 (83%) of 94 with non-vaccine type disease; 915 (75%) of 1219 controls had received at least one dose of PCV10. 94 (30%) of 316 cases were up to date for PCV10 according to age—32 (34%) with vaccine-type disease, 22 (23%) with vaccine-related disease, and 40 (43%) with non-vaccine type disease; 521 (43%) controls were up-to-date for PCV10. Only two children had received more than the recommended number of doses of PCV10, including one child aged 17 months at the time of PCV10 introduction who had received two doses (but should have received one dose as per the catch-up schedule), and one child aged 9 months at the time of introduction who had received four doses (but should have received three doses as per the catch-up schedule).
The adjusted effectiveness of an up-to-date schedule for PCV10 against vaccine-type disease was 83·8% (95% CI 65·9 to 92·3), and against vaccine-related disease 77·9% (41·0 to 91·7; table 3). Protection against non-vaccine type disease was not significant (37·5%, –65·4 to 76·4; table 3). The effectiveness of at least one dose of vaccine was 81·9% against vaccine-type disease (64·4 to 90·8; table 3), and 74·1% against vaccine-related disease (38·3 to 89·1). The effectiveness of a single dose of PCV10 against vaccine-type disease in children aged 12–23 months at the time of vaccine introduction was 68·0% (17·6 to 87·6; table 3); a single dose was not effective against vaccine-related disease. The point estimates for the adjusted effectiveness for at least two, exactly three, and at least three doses were higher than 95%, whereas the effectiveness for exactly four doses was 67·7% but not significant (table 3); of note, only one discordant case-control set contributed to the model for four doses. The adjusted effectiveness of PCV10 against meningitis and pneumonia or bacteraemia vaccine-type disease was similar (87·7% and 81·3%, respectively; table 3). We noted significant protection against serotypes 14 (87·7%, 60·8 to 96·1), 6B (82·8%, 23·8 to 96·1), and 19A (82·2%, 10·7 to 96·4; table 3). Estimates of effectiveness against vaccine serotype 23F, vaccine-related serotype 6A, and non-vaccine serotype 3 were not significant for the up-to-date PCV10 dose schedule by age (table 3), or for at least two and at least three doses against these individual serotypes (data not shown). Use of a lower threshold to identify confounders (changes of ≥10% rather than ≥20% in the odds ratio or β) did not change the direction of associations or significance of the findings (data not shown). The adjusted effectiveness of an up-to-date PCV10 schedule against the PCV7 serotypes was 83·2% (64·7 to 92·1; table 3).
Table 3:
PCV10 effectiveness against invasive pneumococcal disease outcomes in cases (n=316) and controls (n=1219) in the eff ectiveness analysis
| Exposure* | Contributing strata† | Crude effectiveness (95% CI) | Adjusted effectiveness (95% CI)‡ | |
|---|---|---|---|---|
| Overall | ||||
| Vaccine-type invasive pneumococcal disease§ | Up to date for age for number of PCV10 doses | 61/147 | 86·5% (73·2 to 93·2) | 83·8% (65·9 to 92·3) |
| Vaccine-related invasive pneumococcal disease¶ | Up to date for age for number of PCV10 doses | 21/75 | 83·7% (58·7 to 93·6) | 77·9% (41·0 to 91·7) |
| Non-vaccine-type invasive pneumococcal disease|| | Up to date for age for number of PCV10 doses | 18/94 | 25·4% (−79·2 to 68·9) | 37·5% (−65·4 to 76·4) |
| Children eligible for one catch-up dose at 12–23 months ** | ||||
| Vaccine-type invasive pneumococcal disease | One dose | 29/44 | 70·3% (24·0 to 88·4) | 68·0% (17·6 to 87·6) |
| Vaccine-related invasive pneumococcal disease | One dose | 11/15 | 51·0% (−103·1 to 88·2) | 40·6% (−190·2 to 87·8) |
| Non-vaccine-type invasive pneumococcal disease | One dose | 6/10 | −94·9% (−1047·3 to 66·9) | −72·6% (−972·1 to 72·2) |
| Overall by number of doses†† | ||||
| Vaccine-type invasive pneumococcal disease | At least one dose | 78/147 | 83·7% (70·1 to 91·2) | 81·9% (64·4 to 90·8) |
| Vaccine-type invasive pneumococcal disease | Two doses | 15/124 | 90·5% (72·4 to 96·7) | 89·9% (64·1 to 96·6) |
| Vaccine-type invasive pneumococcal disease | At least two doses | 17/124 | 96·6% (88·6 to 99·0) | 95·9% (84·0 to 98·9) |
| Vaccine-type invasive pneumococcal disease | Three doses | 4/108 | 97·5% (87·2 to 99·5) | 96·4% (80·2 to 99·3) |
| Vaccine-type invasive pneumococcal disease | At least three doses | 5/108 | 96·7% (86·1 to 99·2) | 95·4% (78·1 to 99·0) |
| Vaccine-type invasive pneumococcal disease | Four doses | 1/80 | 73·5% (−20·4 to 94·2) | 67·7% (−58·0 to 93·4) |
| Overall by clinical syndrome | ||||
| Pneumonia or bacteraemia (vaccine-type) | Up to date for age for number of PCV10 doses | 26/75 | 88·2% (67·1 to 95·7) | 81·3% (46·9 to 93·4) |
| Meningitis (vaccine-type) | Up to date for age for number of PCV10 doses | 35/72 | 85·1% (61·6 to 94·2) | 87·7% (61·4 to 96·1) |
| Invasive pneumococcal disease due to individual serotypes | ||||
| 14 | Up to date for age for number of PCV10 doses | 29/72 | 87·2% (61·8 to 95·7) | 87·7% (60·8 to 96·1) |
| 6B | Up to date for age for number of PCV10 doses | 11/32 | 87·5% (47·2 to 97·1) | 82·8% (23·8 to 96·1) |
| 19A | Up to date for age for number of PCV10 doses | 9/26 | 90·2% (56·5 to 97·8) | 82·2% (10·7 to 96·4) |
| 3 | Up to date for age for number of PCV10 doses | 9/28 | 5·5% (−278·4 to 76·4) | 7·8% (−271·9 to 77·1) |
| 6A | Up to date for age for number of PCV10 doses | 6/24 | 36·3% (−184·4 to 85·7) | 14·7% (−311·6 to 82·3) |
| 23F | Up to date for age for number of PCV10 doses | 9/18 | 85·6% (7·7 to 97·7) | 57·8% (−336·7 to 95·9) |
| PCV7 serotypes‡‡ | Up to date for age for number of PCV10 doses | 61/146 | 86·5% (73·2 to 93·2) | 83·2% (64·7 to 92·1) |
PCV10=ten-valent pneumococcal conjugate vaccine. PCV7=seven-valent pneumococcal conjugate vaccine.
Reference used to calculate odds ratio for all exposures was zero doses.
Only strata in which cases and controls had discordant vaccination status contributed to conditional logistic regression models; denominator is the overall number of case-control strata in the subgroup and numerator is the number in the strata with discordance (eg, case vaccinated and at least one control not vaccinated, or case not vaccinated and at least one control vaccinated).
Adjusted for receipt of at least one dose of tetravalent (diphtheria-tetanus-pertussis-Haemophilus influenzae type B) vaccine and any chronic illness.
Includes serotypes 1, 4, 5, 6B, 7F, 9V, 14, 18C, 19F, and 23F.
Includes serotypes not in the vaccine that are of the same serogroup as a vaccine-type.
Includes serotypes that are not vaccine-type or vaccine-related.
Age at least 12 months when PCV10 introduced in the state the child was residing in; eligible for one dose: 69 cases and 261 controls.
The analysis of effectiveness by number of doses does not distinguish between different types of doses (ie, primary, booster or catch-up doses).
PCV7 serotypes include 4, 6B, 9V, 14, 18C, 19F, and 23F.
Discussion
PCV10 was effective for routine immunisation of infants in a large national programme in a middle-income country. Results of this study are consistent with those from the only randomised trial of PCV10 against a clinical outcome that showed high efficacy against invasive pneumococcal disease,13 and with prelicensure studies using serological correlates of protection.2 Although few children had received the full four-dose schedule in Brazil, we noted that three or more doses of PCV10 were more than 95% effective against vaccine-type invasive disease (table 3). We noted similar effectiveness against vaccine-type pneumococcal meningitis and pneumonia or bacteraemia (>81%; table 3). Our findings provide insight into the serotype-specific effectiveness of the vaccine against serotypes 14 and 6B, the two most common vaccine serotypes in the cases enrolled in Brazil (figure) and for vaccine-related serotypes, particularly serotype 19A.
PCV10 was initially licensed based on results from studies showing non-inferiority for immunological correlates of protection against the seven serotypes in PCV7.14 Although PCV10 induced adequate antibody concentrations against eight of ten vaccine serotypes, responses to serotypes 6B and 23F were less than the established correlates of protection.2,15 However, PCV10 induced robust opsonophagocytic activity, a measure of antibody function, against all ten antigens.2,15 Clinical correlates of protection for opsonophagocytic assays are not well established. The results of this case-control study provide an important link between serological markers of protection and clinical outcomes. Our results confirm that PCV10 is highly protective against invasive disease due to vaccine-serotypes as a group. Because we enrolled no cases of disease due to serotypes 1 or 5, and only one due to 7F, our estimates of protection against vaccine-type disease essentially indicate PCV10 effectiveness against PCV7 serotypes.
Results of pre-licensure immunogenicity studies also suggested that PCV10 might provide cross-protection against serotype 19A.2,16,17 Although PCV7, which also includes 19F, does not protect against 19A disease,18 the differences in the PCV10 conjugation process might enhance the cross-protective immunological response against serotype 19A.19,20 This serotype was not predominant in Brazil before PCV10 introduction;21,22 however, during the study it was the third most common serotype detected (figure). Thus, the protection shown against this serotype is important in the Brazilian context. Validation of this finding in other settings is important because the point estimate of effectiveness against serotype 19A disease is higher than what might be expected based on immunogenicity data, and the 95% CI was wide. Additionally, PCV10 has not reduced 19A nasopharyngeal carriage in Kenya, where it was introduced in early 2011 (Hammitt L, Johns Hopkins Bloomberg School of Public Health, personal communication).
We noted no significant cross-protection for serotype 6A, which contrasts with pre-licensure immunogenicity data suggesting that PCV10 would confer some protection against that serotype.2,16 However, because the number of cases of 6A disease was small (n=24; figure), these results should be interpreted with caution. The numbers of cases of other serotypes in the vaccine-related group (6C, 6D, 9N, 18A, 18B, and 23A) were too few to assess serotype-specific effectiveness. Further study is needed to quantify PCV10 cross-protection against individual vaccine-related serotypes.
Brazil introduced PCV10 using a single catch-up dose for children aged 12–23 months at the time of introduction. Catch-up campaigns can protect larger numbers of children soon after PCV introduction and might hasten the development of herd protection.23 The effectiveness of a single PCV10 dose in toddlers was not known when the vaccine was introduced in Brazil. The results of a study of a nine-valent PCV had shown that toddlers receiving a single dose had a similar post-primary immune response as toddlers receiving two doses for some serotypes, but importantly not for serotypes 6B, 14, 19F, and 23F,24 which are some of the most common serotypes in Brazil. PCV7 effectiveness against vaccine-type invasive disease was similar when a one-dose and two-dose catch-up schedule was used in children aged 12–23 months.18 In our study, a single dose of PCV10 provided significant protection against vaccine-type disease in this age group (table 3).
Our study had several limitations. Enrolment of cases of invasive pneumococcal disease depended on laboratory capacity for pneumococcal identification at only a few hospitals in ten of 27 states in Brazil; thus, the results might not be representative of the diversity of pneumococcal disease throughout Brazil. Because case detection relied on a well established meningitis surveillance system, meningitis cases are over-represented. Meningitis is the most severe manifestation of pneumococcal disease, as shown by the high mortality rate (36%; table 1) in children with meningitis in this study, but it is also the least common invasive syndrome worldwide.1 Nonetheless, we noted similar levels of protection against vaccine-type meningitis and non-meningitis disease. Misclassification of vaccination status is a concern in observational studies of vaccine effectiveness. However, we obtained written documentation of immunisations for all study participants, likely indicating efforts in Brazil to improve distribution and retention of vaccine cards.25 Also, although we adjusted for important measured confounders in the analysis, children who are vaccinated might differ from those who are not vaccinated in ways that are related to risk of invasive pneumococcal disease and might be difficult to measure.
The findings of this study will aid in the interpretation of other ongoing investigations of the effects of PCV10 introduction in Brazil, including trends in invasive disease, pneumonia,26 and pneumococcal nasopharyngeal carriage,27 adding to the international experience with PCV10 against clinical outcomes (panel).13,28,29 We show that PCV10 as used in the national immunisation programme in Brazil is highly effective against invasive disease caused by vaccine serotypes. We also report significant effectiveness against vaccine-related serotypes, and show important protection from age-appropriate vaccination with a four-dose vaccination schedule—findings that are consistent with other studies of PCV.13,18 These data provided evidence for the Ministry of Health of the benefits of PCV10 use in Brazil and contribute to our understanding of PCV10 effect in routine immunisation programmes. Together with emerging data for PCV13 effects, the results of this study can help inform policy decisions about pneumococcal vaccination in children in countries that have not yet introduced PCV.30
Supplementary Material
Panel: Research in context.
Systematic review
We searched PubMed for reports published before Jan 31, 2014, with the search terms “ten-valent pneumococcal conjugate vaccine”, “pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine”, or “PHiD-CV”, and “effectiveness”, “efficacy”, “impact”, or “invasive pneumococcal disease”. We identified one cluster randomised trial13 of ten-valent pneumococcal conjugate vaccine (PCV10) in which vaccine effectiveness was reported to be 100% (95% CI 83–100) and 92% (58–100) for three and two primary doses, respectively, plus a booster in the second year of life for the prevention of invasive pneumococcal disease due to vaccine serotypes. We also reviewed an abstract29 (obtained from the authors) for a randomised, double-blind study of PCV10 in which vaccine efficacy was reported to be 100% (77–100) against invasive disease due to vaccine serotypes.
Observational studies are needed to assess the effectiveness of PCV10 in routine childhood immunisation programmes and to ascertain protection against individual serotypes.
Interpretation
In our study, vaccination was highly effective against invasive disease caused by vaccine serotypes and provided significant protection against vaccine-related serotypes as a group and against serotype 19A, related to vaccine serotype 19F. The results of this study contribute to reports that show that pneumococcal conjugate vaccines are immunogenic and effective against disease caused by vaccine serotypes. Ongoing surveillance and further studies are needed to assess the effect of vaccination on pneumococcal disease and ascertain vaccine effectiveness against vaccine-related serotypes. Two pneumococcal conjugate vaccines are licensed for routine immunisation of children and are recommended by WHO for inclusion in national immunisation programmes. The results of this study show the effectiveness of the ten-valent pneumococcal conjugate vaccine against invasive pneumococcal disease, supporting the recommendation for its use.
Acknowledgments
Surveillance of invasive pneumococcal disease was funded by the Brazilian Ministry of Health, with support from the Pan American Health Organization. Support for the national reference laboratory for invasive bacterial diseases was provided by the Pan American Health Organization through the regional surveillance for new vaccines (SIREVA II) project. We thank the children and their parents whose participation made this study possible; surveillance units, hospital staff, meningitis and pneumonia surveillance personnel, and public health laboratory staff at the local, state, and federal levels; and Cyndy Whitney (US Centers for Disease Control and Prevention) for valuable input to the manuscript. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Brazilian Pneumococcal Conjugate Vaccine Effectiveness Study Group:
Regina Coeli Magalhães Rodrigues, Marluce Aparecida Assunção Oliveira, Tani Maria Schilling Ranieri, Gladys Maria Zubaran, Ana Lídia Lima Solon, Maria Iracema de Aguiar Patrício, Maria Elisa Paula de Oliveira, Rita de Cássia Vilasboas Silva, Marlene Sera Wille, Pilar Gomes Martinez, Helena Keico Sato, Maria Cristina Hereny Bordim, Luzia Auxiliadora Careli, Vera Lúcia da Glória Malheiros, Zenize Rocha da Silva Costa, Maria Goretti Varejão da Silva, Cleidiane Santos Rodrigues, Ataiza César Vieira, Lucila Tacacô Watanabe, Glaucia Gama Rahal Aires, Robmary Matias de Almeida, Diana Felicia de Araújo Margarido, Ana Lúcia Stone de Souza, Samanta C G Almeida, Angela P Brandão, Lincoln S Prado, Maria Luiza L S Guerra, Orlando Cesar Mantese, Eitan Berezin, Cicero Dias, Cristiana Nascimento, Joice Reis, Ana Lucia Andrade, Solange Andrade, Flavia Lobo, Camile de Moraes, Eliane Castro de Barros, Márcia Lopes de Carvalho, Elias Duarte Gonçalves Correia, and Selma Lina Suzuki.
Footnotes
See Online for an podcast with Jennifer Verani
Declaration of interests
MCdCB has received consulting fees from Pfizer, GlaxoSmithKline, Sanofi Pasteur, and Novartis, and travel grants from Pfizer and GlaxoSmithKline. The other authors declared that they have no competing interests.
Contributor Information
Carla Magda Allan S Domingues, National Immunization Program, Secretariat for Health Surveillance, Ministry of Health, Brasília, Brazil; Center for Tropical Medicine, University of Brasília, Brasília, Brazil.
Jennifer R Verani, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, GA, USA.
Ernesto Issac Montenegro Renoiner, National Immunization Program, Secretariat for Health Surveillance, Ministry of Health, Brasília, Brazil.
Maria Cristina de Cunto Brandileone, National Reference Laboratory for Meningitis and Pneumococcal Infections, Bacteriology Center, Adolfo Lutz Institute, Secretary of Health of the State of São Paulo, São Paulo, Brazil.
Brendan Flannery, Pan American Health Organization, Brasília, Brazil.
Lucia Helena de Oliveira, Pan American Health Organization, Washington, DC, USA.
João Barberino Santos, Center for Tropical Medicine, University of Brasília, Brasília, Brazil.
José Cássio de Moraes, Department of Social Medicine, School of Medical Sciences of Santa Casa, São Paulo, Brazil.
References
- 1.O’Brien KL, Wolfson LJ, Watt JP, et al. , for the Hib and Pneumococcal Global Burden of Disease Study Team. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 2009; 374: 893–902. [DOI] [PubMed] [Google Scholar]
- 2.Vesikari T, Wysocki J, Chevallier B, et al. Immunogenicity of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) compared to the licensed 7vCRM vaccine. Pediatr Infect Dis J 2009; 28 (4 suppl): S66–76. [DOI] [PubMed] [Google Scholar]
- 3.World Bank. World development indicators 2010. http://databank.worldbank.org/data/views/reports/tableview.aspx?isshared=true&ispopular=country&pid=4 (accessed Jan 12 2014).
- 4.Brandileone MC, Vieira VS, Casagrande ST, et al. Prevalence of serotypes and antimicrobial resistance of Streptococcus pneumoniae strains isolated from Brazilian children with invasive infections. Pneumococcal Study Group in Brazil for the SIREVA Project. Regional System for Vaccines in Latin America. Microb Drug Resist 1997; 3: 141–46. [DOI] [PubMed] [Google Scholar]
- 5.Pan American Health Organization. SIREVA II (Sistema de Redes de Vigilancia de los Agentes Responsables de Neumonias y Meningitis Bacterianas) 2013 http://www.paho.org/hq/index.php?option=com_content&view=category&layout=blog&id=3609&Itemid=3953 (accessed Aug 30 2013).
- 6.Brazilian Minstry of Health. Administrative rule number 104 2011 http://bvsms.saude.gov.br/bvs/saudelegis/gm/2011/prt0104_25_01_2011.html (accessed Jan 12, 2014).
- 7.Brazilian Ministry of Health. Information system for notifiable diseases 2009. http://dtr2004.saude.gov.br/sinanweb/ (accessed Jan 12, 2014). [Google Scholar]
- 8.Carvalho Mda G, Tondella ML, McCaustland K, et al. Evaluation and improvement of real-time PCR assays targeting lytA, ply, and psaA genes for detection of pneumococcal DNA. J Clin Microbiol 2007; 45: 2460–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. PCR deduction of pneumococcal serotypes http://www.cdc.gov/ncidod/biotech/strep/pcr.htm (accessed Sept 5, 2013).
- 10.Pimenta FC, Roundtree A, Soysal A, et al. Sequential triplex real-time PCR assay for detecting 21 pneumococcal capsular serotypes that account for a high global disease burden. J Clin Microbiol 2013; 51: 647–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Menezes AP, Reis JN, Ternes YM, et al. Update of pneumococcal PCR serotyping assay for detection of a commonly occurring type 19F wzy variant in Brazil. J Clin Microbiol 2013; 51: 2470–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brazilian Minstry of Health. REDE Interagencial de Informação para a Saúde (2011) Basic health indicators in Brazil 2011. http://tabnet.datasus.gov.br/cgi/idb2011/matriz.htm (accessed Nov 5, 2013). [Google Scholar]
- 13.Palmu AA, Jokinen J, Borys D, et al. Effectiveness of the ten-valent pneumococcal Haemophilus influenzae protein D conjugate vaccine (PHiD-CV10) against invasive pneumococcal disease: a cluster randomised trial. Lancet 2013; 381: 214–22. [DOI] [PubMed] [Google Scholar]
- 14.Dagan R, Frasch C. Clinical characteristics of a novel 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine candidate (PHiD-CV). Introduction. Pediatr Infect Dis J 2009; 28 (4 suppl): S63–65. [DOI] [PubMed] [Google Scholar]
- 15.Prymula R, Schuerman L. 10-valent pneumococcal nontypeable Haemophilus influenzae PD conjugate vaccine: Synflorix. Expert Rev Vaccines 2009; 8: 1479–500. [DOI] [PubMed] [Google Scholar]
- 16.Wysocki J, Tejedor JC, Grunert D, et al. Immunogenicity of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) when coadministered with different Neisseria meningitidis serogroup C conjugate vaccines. Pediatr Infect Dis J 2009; 28 (4 suppl): S77–88. [DOI] [PubMed] [Google Scholar]
- 17.Bermal N, Szenborn L, Edison A, et al. Safety and immunogenicity of a booster dose of the 10-valent pneumococcal nontypeable Haemophilus influenzae protein D conjugate vaccine coadministered with DTPw-HBV/Hib and poliovirus vaccines. Pediatr Infect Dis J 2011; 30: 69–72. [DOI] [PubMed] [Google Scholar]
- 18.Whitney CG, Pilishvili T, Farley MM, et al. Effectiveness of seven-valent pneumococcal conjugate vaccine against invasive pneumococcal disease: a matched case-control study. Lancet 2006; 368: 1495–502. [DOI] [PubMed] [Google Scholar]
- 19.Mrkvan T, Hoet B, Adegbola RA, Van Dyke MK, Hausdorff WP. Serotype 19A and the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV): Lessons learned to date Milan: European Society for Paediatric Infectious Diseases (ESPID), 2013. [Google Scholar]
- 20.Poolman J, Frasch C, Nurkka A, Käyhty H, Biemans R, Schuerman L. Impact of the conjugation method on the immunogenicity of Streptococcus pneumoniae serotype 19F polysaccharide in conjugate vaccines. Clin Vaccine Immunol 2011; 18: 327–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brandileone MC, Casagrande ST, Guerra ML, Zanella RC, Andrade AL, Di Fabio JL. Increase in numbers of beta-lactam-resistant invasive Streptococcus pneumoniae in Brazil and the impact of conjugate vaccine coverage. J Med Microbiol 2006; 55(pt 5): 567–74. [DOI] [PubMed] [Google Scholar]
- 22.Brandileone MC, de Andrade AL, Di Fabio JL, Guerra ML, Austrian R. Appropriateness of a pneumococcal conjugate vaccine in Brazil: potential impact of age and clinical diagnosis, with emphasis on meningitis. J Infect Dis 2003; 187: 1206–12. [DOI] [PubMed] [Google Scholar]
- 23.Melegaro A, Choi YH, George R, Edmunds WJ, Miller E, Gay NJ. Dynamic models of pneumococcal carriage and the impact of the heptavalent pneumococcal conjugate vaccine on invasive pneumococcal disease. BMC Infect Dis 2010; 10: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldblatt D, Southern J, Ashton L, et al. Immunogenicity and boosting after a reduced number of doses of a pneumococcal conjugate vaccine in infants and toddlers. Pediatr Infect Dis J 2006; 25: 312–19. [DOI] [PubMed] [Google Scholar]
- 25.Barata RB, Ribeiro MC, de Moraes JC, Flannery B. Socioeconomic inequalities and vaccination coverage: results of an immunisation coverage survey in 27 Brazilian capitals, 2007–2008. J Epidemiol Community Health 2012; 66: 934–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Afonso ET, Minamisava R, Bierrenbach AL, et al. Effect of 10-valent pneumococcal vaccine on pneumonia among children, Brazil. Emerg Infect Dis 2013; 19: 589–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neves FP, Pinto TC, Correa MA, et al. Nasopharyngeal carriage, serotype distribution and antimicrobial resistance of Streptococcus pneumoniae among children from Brazil before the introduction of the 10-valent conjugate vaccine. BMC Infect Dis 2013; 13: 318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Wals P, Lefebvre B, Defay F, Deceuninck G, Boulianne N. Invasive pneumococcal diseases in birth cohorts vaccinated with PCV-7 and/or PHiD-CV in the province of Quebec, Canada. Vaccine 2012; 30: 6416–20. [DOI] [PubMed] [Google Scholar]
- 29.Tregnaghi MW, Sáez-Llorens X, López P, et al. Efficacy of 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) against invasive pneumococcal disease in Latin America 9th International Symposium on Antimicrobial Agents and Resistance; Kuala Lumpur, Malaysia; March 13–15, 2013; 2013. [Google Scholar]
- 30.WHO. Pneumococcal vaccines WHO position paper–2012. Wkly Epidemiol Rec 2012; 87: 129–44. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


