Figure 3: Schematic representation of Notch receptor activation.
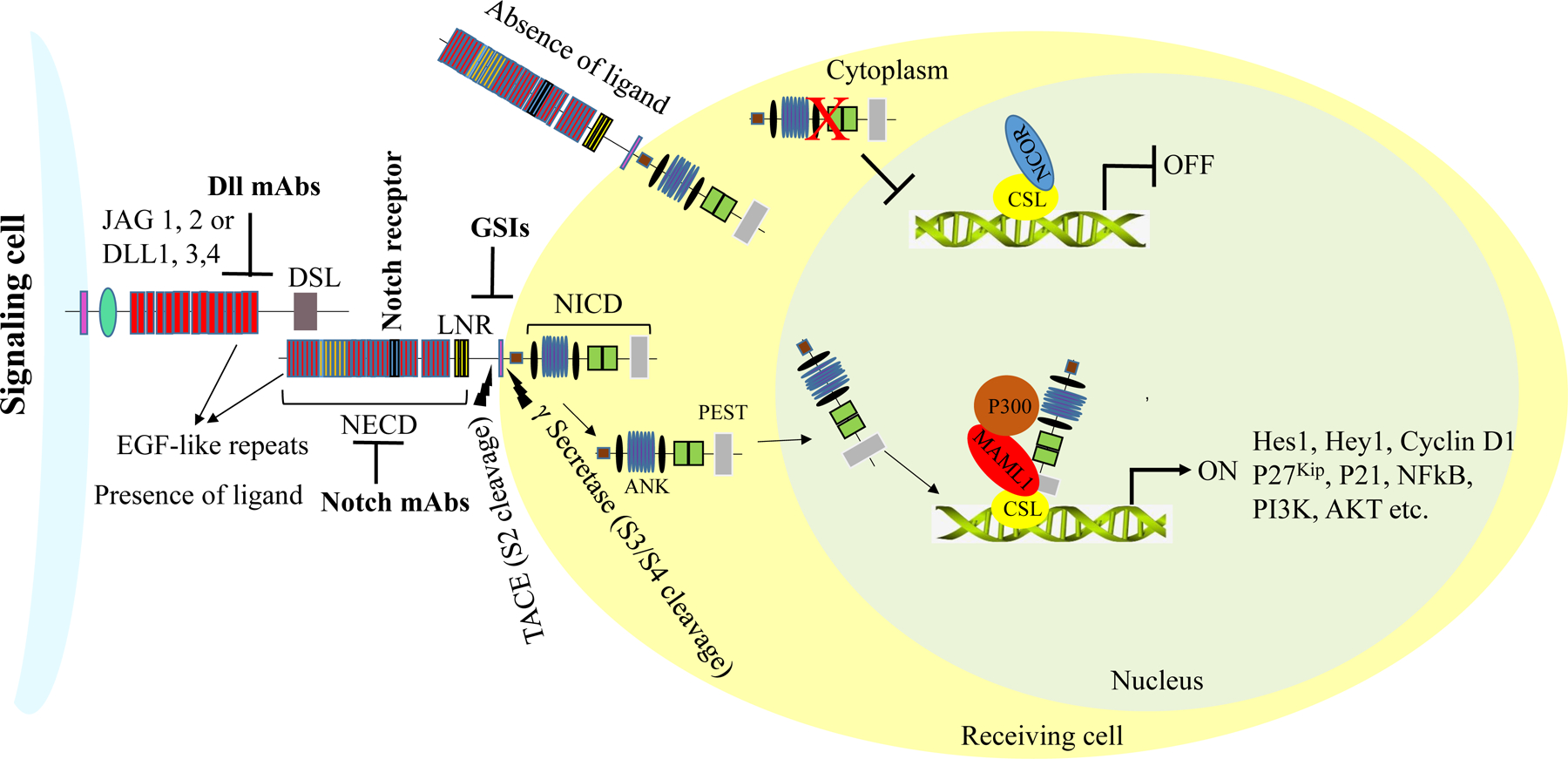
Notch receptors are activated upon binding to Serrate- and Delta-like ligands present on the cell membranes of adjacent cells. Following successful activation, Notch receptors undergo a series of proteolytic cleavages at site 2 (S2), mediated by metalloprotease 10 (ADAM10) and TACE (TNFα converting enzyme). Additional proteolytic cleavages at the transmembrane domain (NTD) are carried out by a multi-subunit complex, γ-secretase, at site 3 (S3). The Notch intracellular domains (NICDs) are then released into the cytoplasm. The NICDs further translocate into the nucleus, where they displace histone deacetylase and co-repressors in CSL repressor complexes and recruit MAML1 and histone acetyltransferase p300 to form active transcriptional complexes, which regulate the transcriptional activity of Notch target genes.
