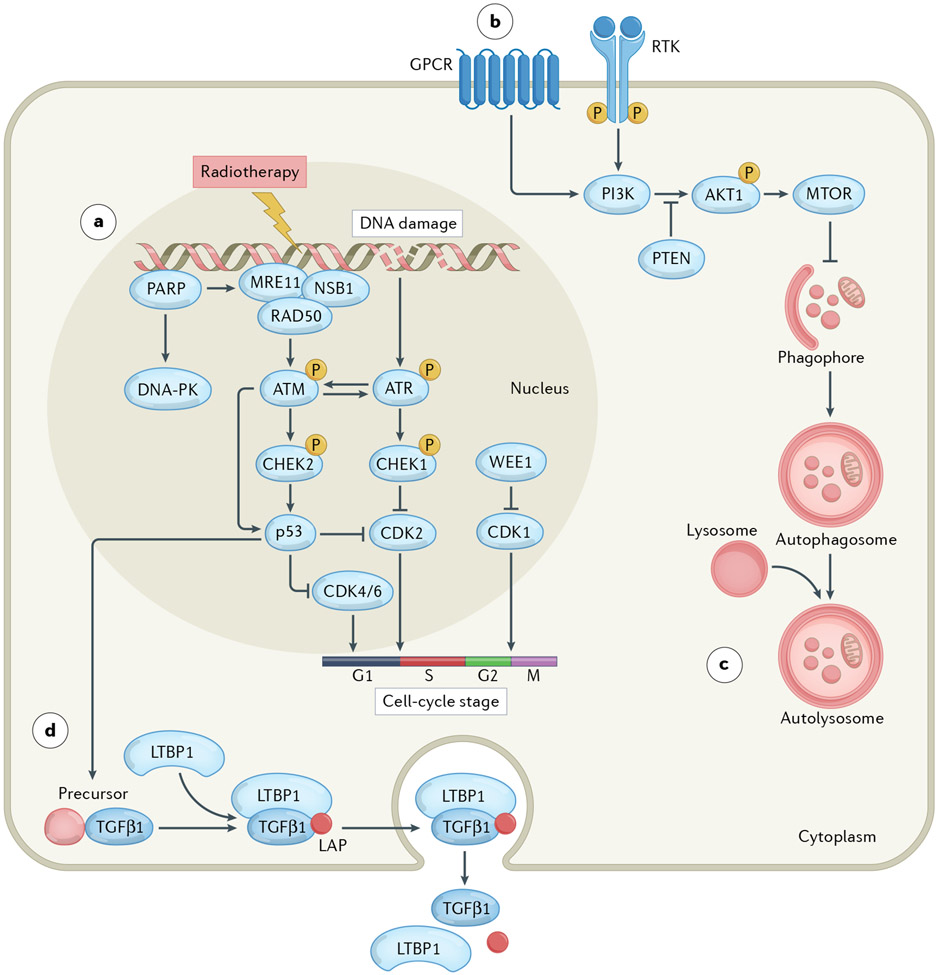
Fig. 1 ∣. Cytoprotective pathways elicited by radiotherapy.
Ionizing radiation damages a variety of macromolecules including nuclear DNA, either directly or upon generation of reactive oxygen species. Such damage is often detected by a molecular complex encompassing meiotic recombination 11 (MRE11), DNA repair protein Rad50 (RAD50) and Nijmegen breakage syndrome 1 (NSB1) in co-operation with members of the poly(ADP-ribose) polymerase (PARP) protein family. Formation of this complex results in the sequential activation of ATM, CHEK2 and p53. Alternatively or concomitantly, the DNA damage induced by radiotherapy drives the activation of ATR and consequently CHEK1, DNA-dependent protein kinase (DNA-PK) or WEE1 signalling. Ultimately, these pathways converge on the inhibition of cyclin-dependent kinases (CDKs) resulting in arrested cell-cycle progression at specific checkpoints, which enables DNA repair and hence supports radioresistance (part a). The DNA damage response elicited by radiotherapy also promotes (directly or indirectly) the hyperactivation of PI3K signalling, resulting in the delivery of cytoprotective signals via AKT1 and MTOR (part b), the activation of autophagy (which is generally under negative regulation by MTOR) (part c), as well as the synthesis, secretion and activation of transforming growth factor-β1 (TGFβ1) (part d). GPCR, G-protein-coupled receptor; LAP, latency associated peptide; LTBP1, latent transforming growth factor-β binding protein 1; PTEN, phosphatase and tensin homologue; RTK, receptor tyrosine kinase.