Abstract
The current study investigated the impact of different concentrations of purified egg yolk immunoglobulin Y (IgY) supplemental food on the growth performance, behaviors, cecal contents of Escherichia coli, and the meat quality of broiler chicks. Four dietary groups were given to 180 female Ross broiler chicks at random (n = 45 for each). The control group was fed a standard diet only, whereas the other three experimental groups were fed the same basic diet supplemented with 1,500, 3,000, and 4,000 μg/ml IgY for a duration of 42 days. Significant greater behavioral activities, including, feeding, drinking, and dust bathing (p < 0.05), in the birds fed 4,000 μg/ml of IgY compared to the control group were observed. Greater weight gains of the crop, proventriculus, gizzard, and intestine (p < 0.05) were observed for broiler chicks fed 4,000 μg/ml of IgY when compared to the control group. After 3 weeks of feeding, the groups fed 3,000 and 4,000 μg/ml IgY had significant lower E. coli counts in the muscle and cecal contents (p < 0.05) when compared to the control group. Moreover, dietary supplementation with 4,000 μg/ml IgY in the third week and 3,000 μg/ml IgY in the sixth week resulted in greater weight gain (p < 0.01) when compared to the control group. Also, at week 3, chicks fed 4,000 μg/ml of IgY had a lower feed conversion ratio (FCR) when compared to the control group (p < 0.05). At week 6, chicks fed 3,000 μg/ml of IgY had lower FCR than the control (p < 0.05). The circulating heterophile/lymphocyte ratio was simply altered in birds fed variable IgY concentrations (1,500, 3,000, and 4,000 μg/ml), with no significant differences compared to the control group due to the individual resistance of each bird to physiological stress. The addition of 4,000 μg/ml IgY to the diet enhanced the nutritive value of meat, including protein, fat, and ash content (p < 0.05). Our study concluded that dietary supplementation of 3,000 and/or 4,000 μg/ml IgY improved the growth rates, behavioral activities, intestinal health indices, and meat quality of broiler chicks.
Keywords: immunoglobulin Y, broiler, performance, behavior, E. coli, meat quality
Introduction
During the last decade, researchers have worked to enhance the hygienic conditions of broiler farms. This would be possible by moving away from using antimicrobial drugs, which are often found in poultry products such as meat and eggs as harmful residues, negatively affecting the health of human beings (1). Furthermore, owing to the contamination of carcasses during evisceration and insufficient poultry raising circumstances, the prevalence of intestinal colonization in chickens caused by the pathogenic Escherichia coli is frequently common, presenting an elevated danger to humans (2, 3). Egg yolk immunoglobulin Y (IgY) is a safe and effective alternative to drugs and does not induce allergic responses. This is due to the unique structure of the IgY fragment crystallization (FC) region, which prevents the Fc receptors from attaching to immune cells (4–7). IgY has attracted a lot of interest in recent years since it is simple to make in large quantities and is both cost-effective and safe (8). Newly hatched chicks receive maternal antibodies from their dams needed for egg protection (9). On the day 7 after hatching, the IgY is reduced as soon as the bird starts developing antibodies (10). Hen egg yolk has 8–20 mg/ml of IgY (11). Recently, we have revealed that broilers fed a novel combination of IgY and probiotics improved their behavioral activities and immunity responses (7). Furthermore, using this combination improved the meat quality characteristics and nutritional value (12). In addition, we determined that 1,500 μg/ml IgY had a bacteriocidal effect on Gram-positive bacteria; however, we could not evaluate which concentration(s) of IgY might be effective in killing Gram-negative bacteria. There is a necessity to adjust the effective concentration in order to kill Gram-negative bacteria. Owing to the beneficial role of IgY, we were curious to study the effects of various concentrations of IgY powder mixed in ration on the bird's behavior, performance and on the physiological parameters, meat nutritive value, and microbiological load of the meat, notably E. coli contamination. Our study is ultimately aimed at producing nutritional and safe meat for human consumption.
Materials and Methods
Study Design, Animals, and Management
One hundred and eighty broiler chicks (female Ross strain, 1.3 ± 0.21 days old, 48.1 ± 0.2 g) were obtained from the Egyptian Nutrivet Animal Health Company. They were randomly divided into four equivalent groups (n = 45) with three replicates and handled up to day 42. Multiple concentrations (0, 1,500, 3,000, and 4,000 μg/ml) of IgY were used. The schematic cartoon in Figure 1 shows the study design of the four experimental groups. The first group, serving as the control, received the basal ration, whereas the second, third, and fourth groups were fed different concentrations of IgY powder (1,500, 3,000, and 4,000 μg/ml, respectively). All chicks were given unlimited source of food and water and were raised under identical environmental and management protocols (7, 12). As shown in Table 1, diet was prepared in the form of corn–soya for all groups. The entire starter, growing, and finisher period diets were balanced according to a previous report (13). All chickens were immunized under routine immunization programs for infectious bronchitis and Newcastle and Gumboro diseases. The body weight (BW), weight gain (WG), feed intake (FI), and feed conversion ratio (FCR) were estimated and then compared among groups on days 21 and 42 (7, 12). Moreover, cecal contents from each bird were collected in sterile tubes individually for analysis (14). All methodologies of animal testing were carried out in line with the animal care and use committee of the Veterinary Medicine Faculty, Mansoura University, Mansoura, Egypt. Protocol dated 20032020 gave its approval to the Animal Experimental Guidelines.
Figure 1.
Schematic cartoon of the study strategy designed using Biorender.com.
Table 1.
Chemical composition of the diet.
| Ingredients | Ration | ||
|---|---|---|---|
| Starter | Grower | Finisher | |
| Crude protein (%) | 23 | 21 | 19 |
| Crude fat (%) | 5.92 | 6.62 | 6.86 |
| Metabolizable energy (kcal/kg) | 3,020 | 3,100 | 3,200 |
| Crude fiber (%) | 3.76 | 3.46 | 3.20 |
Preparation of IgY
Three hundred fertile eggs from hens, purchased from Alarabia Lell-Alaaf, Quesna, Elmenofia governorate, Egypt, were used for isolation and collection of egg yolk IgY. The water dilution (WD) method was used to purify the IgY from egg yolk. It is one of the best precipitation methods. It is a quick and cost-efficient method for isolating IgY from the entire egg yolk (15). The WD method produces the greatest yield (96%) using a salt precipitation cryo-ethanol treatment, followed by the release of the IgY powder by heating, thereby stabilizing the antibody molecules (16). Hen egg yolk regularly has 8–20 mg/ml of IgY (11). Absorbance at 280 nm wavelength using NanoDrop One apparatus and PierceTM Chicken IgY Purification Kit (Thermo Fisher ScientificTM, New York, NY, USA) were used to determine the IgY concentration. The amount of IgY powder was given in a mixture (0.5 g/kg ration) to the chickens starting from 7 days until the slaughtering time based on our previous reports (7, 10). We thought to include IgY in multiple concentrations (1,500, 3,000, and 4,000 μg/ml) in the ration on day 8 because newly hatched chicks would be fed egg yolk IgY on the first days of hatching until the end of the first week of their lives. Consequently, the amount of IgY introduced to birds would be increased daily based on the diet eaten per kilogram
Behavioral and Welfare Assessments
All chicks were carefully managed and reared during optimal weather conditions in addition to ensuring a low-stress environment to maximize welfare. The behavior of the birds in each treatment group was recorded using the scan sampling method for 4 days a week between weeks 2 and 6 after hatching. The behavioral assays were performed four times a day, once in the morning (0700 hours), mid-day (1,200 h), afternoon (1,500 h), and evening (2,000 h) for 1-h sessions each (17). Behavior was evaluated by recording the number of chicks spotted performing different behaviors at a 15-s sample interval (instantaneous sampling) using a computerized camera recording system. The cameras (Hikvision, Binjiang District, Hangzhou, China) were fixed, directly overhead, and recorded in real time. Data were stored on Hikvision digital video recorders for the behavioral analysis. A well-defined ethogram was developed to define the behaviors we were studying. For instance, ingestion includes eating (the bird's head is extended toward the feeder, pecking the available food resources) and drinking (the bird's beak is in contact with water in or above the drinker, and the bird appears to be drinking) behavior. Preening (where the bird's beak contacts the bird's plumage), dust bathing (when the bird uses its wings, head, neck, and legs to bathe in the dust), and wing/leg stretching are all examples of body maintenance (the bird extends wing or leg out from the body), as well as head scratching (the bird scratch his head with toes).
Internal Organ Weight
After slaughtering, the crop, proventriculus, gizzard, and intestine were weighed and calculated as a percentage of dressed carcass weight.
Blood Analysis
All chickens were slaughtered at 42 days of age by decapitation. The values of packed cell volume (PCV, in percent) were then calculated (18). The hemoglobin (Hb, in grams per deciliter) concentration was estimated using a commercial colorimetric kit from Vitro Scientific Company, Egypt (19). In addition, differential leukocytic counts (DLC, in cells per microliter) were estimated based on the morphology and the ratio of each cell. Lastly, we analyzed the heterophil/lymphocyte (H/L, in percent) ratio as an indicator of stress (20).
Antimicrobial Bioactivity Assay
The antimicrobial bioactivity assay was achieved as previously described (16) using the liquid broth method. E. coli were obtained from the Bacteriology Unit, Egyptian Animal Health Research Institute, El-Dokki, Giza. The mid-logarithmic phase of E. coli was collected and resuspended in trypticase soy broth 1% to provide 106-107 colony forming units (CFU)/ml. An equal amount of bacterial suspension and the tested sample were mixed together and incubated in the absence of protein (21–23). The killing power of the IgY concentrations to E. coli was calculated using the equation described previously (24). Briefly, ΔLog killing = log10 nc – Log10 np, where nc and np are CFU per milliliter of mock and treated cells, respectively.
Nutritive Value of Meat
On day 42, moisture was analyzed by oven drying (25), the ash was analyzed by Muffle Furance (26), the protein was analyzed by the Kjeldahl method (25), the fat was analyzed by Soxhlet extractor (27), and thiobarbituric acid (TBA) was analyzed using the Vyncke method (28).
Enumeration of E. Coli in Breast Meat and Cecal Contents
The E. coli counts in breast and thigh meat samples and the cecal contents were evaluated using eosin methylene blue, as previously described (4, 5, 29).
Statistical Analysis
One-way ANOVA using SPSS software, version 16 (30), was used for the analysis of E. coli count in cecal contents and in meat samples. WG, FI, FCR, and the counts of E. coli were examined as the quantitative parameters. Relationships between variables were analyzed using the general linear model to reduce the possibility of random factors introducing variability into the data and also to allow for the intervention approach. We performed the mean differences in groups by way of the Turkey test. A comparison of variables was done using non-parametric tests. We used ANOVA testing because, in general, our results were just normally distributed: fixed (treatments) and random (repetitions) effect factors. The mean effects showed significance when the p-value was <0.05. Moreover, the chi-square test was used to compare the E. coli counts. The F distribution had two parameters, the between-groups degree of freedom, k, and the residual degree of freedom, N – k, represented as the following ANOVA formula: (df1 = k – 1, df2 = N – k), where df is the degree of freedom, k is the number of groups, and N is the number of observations.
Results
Behavioral Activities
There were significant differences in behavioral activity between the groups (Table 2). Supplementation of the poultry diet with IgY powder in concentrations of 3,000 and 4,000 μg/ml had a significant effect on the percentages of scan feeding, drinking, and dust bathing. The addition of IgY powder of 3,000 μg/ml improved the feeding (F(3, 176) = 1.256, p = 0.0004), drinking (F(3, 176) = 1.697, p = 0.02), and dust bathing (F(3, 176) = 1.392, p = 0.002) in comparison with the control group. On the other hand, adding IgY powder at 4,000 μg/ml significantly enhanced the feeding, drinking, and dust bathing (F(3, 176) = 1.123, p = 0.008; F(3, 176) = 1.417, p = 0.02; and F(3, 176) = 1.257, p = 0.002, respectively). Moreover, there was no significant difference in dust bathing among chickens fed 3,000 and/or 4,000 μg/ml IgY, but there was an abundant increase.
Table 2.
Behavioral observations (mean ± SEM) recorded in various experimental groups.
| Control | 1,500 μg/ml IgY | 3,000 μg/ml IgY | 4,000 μg/ml IgY | |
|---|---|---|---|---|
| Feeding | 5.24 ± 0.4 | 8.56 ± 0.3 | 14.90 ± 0.2** | 15.10 ± 0.9** |
| Drinking | 3.32 ± 0.3 | 2.56 ± 0.2 | 7.31 ± 0.6* | 9.49 ± 0.2* |
| Preening | 3.52 ± 0.9 | 3.41 ± 0.3 | 2.39 ± 0.5** | 2.11 ± 0.4** |
| Dust bathing | 0.25 ± 0.08 | 0.62 ± 0.03 | 0.81 ± 0.12 | 0.75 ± 0.05 |
| Wing or leg stretching | 0.44 ± 0.02 | 0.25 ± 0.02 | 0.55 ± 0.03 | 0.61 ± 0.01 |
| Head scratching | 0.11 ± 0.01 | 0.15 ± 0.02 | 0.13 ± 0.05 | 0.12 ± 0.02 |
Data were presented as the mean ± SEM. Statistics was done using one-way ANOVA.
**p <0.01, *p <0.05 (significance level compared to the control group).
Weight of Internal Organs
There was a considerable influence of IgY on the relative organ weight, as shown in Table 3, with the relative weights of the crop, proventriculus, gizzard, and intestine being significantly greater in birds administered IgY at a concentration of 4,000 μg/ml when dressed carcass weight was considered (F(3, 176) = 1.361, p = 0.01; F(3, 176) = 1.123, p = 0.05; F(3, 176) = 1.067, p = 0.05; and F(3, 176) = 1.251, p = 0.05, respectively). However, the weights in the groups supplemented with 1,500 and 3,000 μg/ml IgY were relatively the same, with no significant differences compared to the control group.
Table 3.
Weight of internal organs across the experimental groups.
| Internal organs | Control | 1,500 μg/ml IgY | 3,000 μg/ml IgY | 4,000 μg/ml IgY |
|---|---|---|---|---|
| Crop | 0.23 ± 0.001 | 0.31 ± 0.001 | 0.22 ± 0.02 | 0.35 ± 0.05** |
| Proventriculus | 0.495 ± 0.04 | 0.523 ± 0.02 | 0.561 ± 0.08 | 0.712 ± 0.06* |
| Gizzard | 2.30 ± 0.08 | 2.34 ± 0.06 | 2.44 ± 0.03 | 2.66 ± 0.08* |
| Intestine | 4.54 ± 0.50 | 4.92 ± 0.60 | 5.25 ± 0.30 | 6.08 ± 0.90* |
Statistics (mean ± SEM) was done using one-way ANOVA.
**p <0.01, *p <0.05 (compared to the control).
Growth, Performance Rate, and Mortalities
The growth and performance rates of chickens showed that the mean of BW was relatively similar on day 1. The WG, FI, and FCR for all groups were also the same, and there was no significant difference among groups observed (Table 4). At the end of week 3, we could not find any significant difference in the WG of the control chickens compared to those that received 1,500 μg/ml IgY. However, chicks supplemented with 4,000 μg/ml IgY had the highest WG (F(3, 176) = 1.468, p = 0.005) compared with the controls. Moreover, chickens fed 3,000 and 1,500 μg/ml IgY showed no significant differences. The chickens presented no significant difference in growth among treatment groups by the end of the third week when FI was compared. The FCR for chickens at the end of week 3 was lower than that in the controls (F(3, 176) = 1.351, p = 0.004) at 4,000 μg/ml IgY. When comparing the total FI at week 6 of the growing stage, we found no significant differences. However, the FCR of chickens supplemented with 3,000 and 4,000 μg/ml at the end of week 6 was slightly lower (F(3, 176) = 1.423, p = 0.046 and p = 0.035, respectively) than that of the control chickens and those supplemented with 1,500 μg/ml of IgY powder. Moreover, our result could not record any significant difference in the FCR for chickens that received 3,000 and 4,000 μg/ml IgY; however, the WG at week 6 was improved (F(3, 176) = 1.534, p = 0.004) in the group that received 3,000 μg/ml IgY compared with the controls. Furthermore, only the control group recorded an 8.8 mortality percentage. As shown in Table 5, chicks supplemented with 4,000 μg/ml IgY had the highest BW (F(3, 176) = 1.328, p = 0.000) compared to the controls. In addition, the breast and thigh weights of chickens were the highest when supplemented with 4,000 μg/ml IgY (p = 0.007 and p = 0.033, respectively) compared to the controls. The breast and thigh ratios relative to the BWs were the highest (9.7 and 7.2%, respectively) in chicks supplemented with 4,000 μg/ml IgY compared to the controls.
Table 4.
Growing rates in the experimental groups fed different IgY concentrations.
| Item | Control | IgY concentration | ||
|---|---|---|---|---|
| 1,500 μg/ml IgY | 3,000 μg/ml IgY | 4,000 μg/ml IgY | ||
| Day 1 BW (g) | 48.40 ± 2.08 | 47.60 ± 2.52 | 48.30 ± 2.43 | 48.10 ± 2.05 |
| Week 1 WG (g) | 106.80 ± 3.33 | 106.90 ± 5.55 | 112.20 ± 3.79 | 111 ± 4.14 |
| Week 1 FI (g) | 93.50 ± 6.88 | 90.70 ± 6.22 | 88.70 ± 5.11 | 87 ± 4.44 |
| Week 1 FCR (g) | 0.88 ± 0.11 | 0.85 ± 0.11 | 0.79 ± 0.07 | 0.78 ± 0.02 |
| Week 3 WG (g) | 708 ± 26.30 | 731 ± 24.10 | 735.60 ± 18.40 | 751 ± 25.60** |
| Week 3 FI (g) | 866 ± 18.30 | 861 ± 17.30 | 841 ± 25.20 | 836 ± 25.80 |
| Week 3 FCR (g) | 1.22 ± 0.08 | 1.18 ± 0.01 | 1.14 ± 0.04 | 1.11 ± 0.03* |
| Week 6 WG (g) | 2,125 ± 153 | 2,161 ± 166 | 2,329 ± 181.00** | 2,277 ± 142 |
| Week 6 FI (g) | 3,860 ± 342 | 3,885 ± 263 | 3,716 ± 166.00 | 3,693 ± 154 |
| Week 6 FCR (g) | 1.82 ± 0.11 | 1.80 ± 0.18 | 1.60 ± 0.22* | 1.62 ± 0.13* |
| Mortalities (%) | 8.8 | 0 | 0 | 0 |
Data were presented as the mean ± SEM and evaluated using one-way ANOVA.
BW, body weight; WG, weight gain; FI, feed intake; FCR, feed conversion rate.
**p <0.01, *p <0.05 (significance level compared to the control group).
Table 5.
Weight of the breast and thigh (in grams and percent, relative to bird weight).
| Item | Control | 1,500 μg/ml IgY | 3,000 μg/ml IgY | 4,000 μg/ml IgY |
|---|---|---|---|---|
| BW (g) | 2,099.60 ± 12.30 | 2,160.21 ± 183.05 | 2,243.42 ± 145.91 | 2,475.40 ± 133.20*** |
| Breast W (g) | 159 ± 5.32 | 163 ± 5.78 | 217 ± 9.40 | 241 ± 10.22** |
| Breast (% of BW) | 7.57 | 7.55 | 9.67 | 9.74 |
| Thigh W (g) | 142 ± 4.27 | 151 ± 4.47 | 162 ± 5.38 | 173 ± 6.36* |
| Thigh (% of BW) | 6.14 | 6.99 | 7.13 | 7.23 |
Data were presented as the mean ± SEM and evaluated using one-way ANOVA.
BW, body weight; IgY, immunoglobulin Y; W, weight.
***p <0.001, **p <0.01, *p <0.05 (compared to the control group).
Physiological Analyses
Interestingly, as shown in Table 6, the red blood cell (RBC) counts showed the lowest record (F(3, 176) = 1.357, p = 0.003) in the group fed 4,000 μg/ml of IgY compared to the control group. However, the Hb concentration recorded no significant difference among groups. Also, the PCV ratio of the group fed 4,000 μg/ml of IgY was the lowest rank (F(3, 176) = 1.827, p = 0.035) compared to the controls. Meanwhile, the H/L ratio revealed a similarity in all groups. However, heterophils were the lowest (F(3, 176) = 1.498, p = 0.006), whereas eosinophils were not recorded as significant in the group fed 4,000 μg/ml of IgY. Lymphocytes were more elevated in all IgY-treated groups than in the control group. However, no significant differences were recorded for the basophil and monocyte ratios among all the groups.
Table 6.
Blood parameters of broiler carcasses fed multiple IgY concentrations at day 42.
| Blood analysis | Treatments | |||
|---|---|---|---|---|
| Control (no IgY) | 1,500 μg/ml IgY | 3,000 μg/ml IgY | 4,000 μg/ml IgY | |
| RBCs (cells/μl) | 190 ± 4.32 | 202 ± 9.44 | 190 ± 6.88 | 183 ± 1.66** |
| Hb conc. (g/dl) | 15.11 ± 1.27 | 15.54 ± 0.98 | 15.32 ± 0.75 | 16.19 ± 1.38 |
| PCV (%) | 31.66 ± 0.65 | 33.66 ± 1.23 | 31.66 ± 0.83 | 30.5 ± 0.19* |
| H/L ratio | 0.51 ± 0.09 | 0.94 ± 0.98 | 1.04 ± 1.21 | 0.69 ± 1.27 |
| Heterophils (cells/μl) | 39.50 ± 4.04 | 43.42 ± 3.66 | 42.37 ± 3.74 | 27 ± 7.54** |
| Lymphocytes (cells/μl) | 57.75 ± 3.25 | 50.42 ± 3.44 | 47.75 ± 3.87 | 64.66 ± 6.77 |
| Basophils (cells/μl) | 0.25 ± 0.14 | 0.14 ± 0.12 | 0.50 ± 0.18 | 0.0 ± 0.28 |
| Monocytes (cells/μl) | 6.50 ± 1.32 | 5.85 ± 0.99 | 8.37 ± 0.98 | 3.33 ± 0.88 |
| Eosinophils (cells/μl) | 1.00 ± 0.61 | 0.14 ± 0.97 | 1.00 ± 0.38 | 0.0 ± 0.14 |
Data were presented as the mean ± SEM and analyzed using one-way ANOVA.
IgY, immunoglobulin Y; RBCs, red blood cells; Hb, hemoglobin; PCV, packed cell volume; H/L, heterophil/lymphocyte ratio.
**p <0.01, *p <0.05.
Assessment of the Antimicrobial Activity of Multiple IgY Concentrations on E. Coli
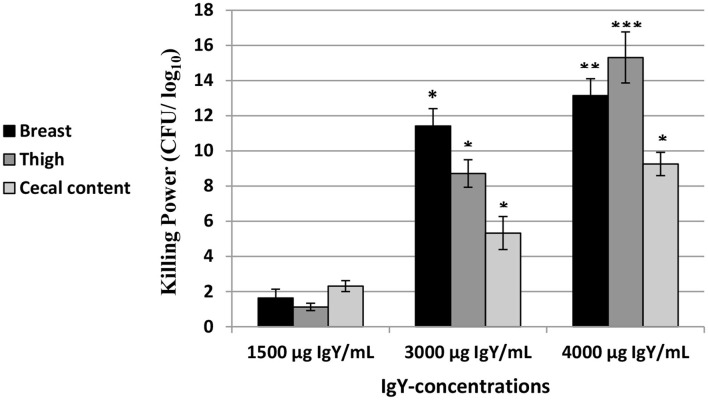
As shown in Figure 2, the killing power (Log10 CFU/ml) of the concentrations of IgY used (1,500, 3,000, and/or 4,000 μg/ml) was confirmed against E. coli in the breast, thigh, and cecal content of chicken. Our results indicated that the highest killing power against E. coli is at 4,000 μg/ml of IgY in the breast (p = 0.032), thigh (p = 0.007), and cecal content (p = 0.007) compared to 1,500 μg/ml of IgY.
Figure 2.
Log10 of the antibacterial activity (mean ± SEM) of the immunoglobulin Y (IgY) concentrations (1,500,3,000,and 4,000 μg/ml) in the breast,thigh,and cecal content of chicken against Escherichia coli. *p < 0.05,**p < 0.01,***p < 0.001 (compared to 1,500 μg/ml IgY).
Assessment of the Level of Meat Contamination With E. Coli
As shown in Table 7, the level of meat contamination with E. coli was studied within various groups. Chickens supplemented with 3,000 μg/ml IgY in their rations significantly had reduced levels of E. coli contamination in the examined breast and thigh muscles (F(3, 176) = 1.527, p = 0.046; F(3, 176) = 1.328, p = 0.039, respectively) in comparison with the control group. Moreover, those fed 4,000 μg/ml IgY had lower levels of E. coli contamination in the examined breast and thigh muscles (F(3, 176) = 1.527, p = 0.031; F(3, 176) = 1.328, p = 0.025, respectively), comparable to the standard control group.
Table 7.
Escherichia coli counts in meat muscle and cecal contents.
| E. coli colony count (Log10 CFU/g) | Control | 1,500 μg/ml IgY | 3,000 μg/ml IgY | 4,000 μg/ml IgY |
|---|---|---|---|---|
| Breast | 7.50 ± 0.20 | 6.80 ± 0.30 | 6.20 ± 0.20* | 6.10 ± 0.30* |
| Thigh | 6.40 ± 0.30 | 6.30 ± 0.30 | 5.80 ± 0.20* | 5.70 ± 0.20* |
| Cecal content | 8.31 ± 2.32 | 7.33 ± 1.82 | 5.15 ± 1.73* | 4.10 ± 1.62* |
Data were presented as the mean ± SEM and evaluated using one-way ANOVA.
*p <0.05.
Enumeration of E. Coli in the Cecal Content
We found that supplementation of 3,000 and 4,000 μg/ml of IgY in the chicken ration significantly reduced E. coli enumeration in the cecum (F(3, 176) = 1.679, p = 0.028 and p = 0.031, respectively). Nevertheless, no significant difference was noticed between bacteria in chicks that received 1,500 μg/ml IgY compared to the controls (Table 7).
Impact of IgY Concentrations on Meat Quality
As shown in Table 8, the protein and ash percentages were significantly greater (F(3, 176) = 1.363, p = 0.0001) than those of the control at 4,000 μg/ml IgY. The fat and moisture contents were significantly reduced at 4,000 μg/ml IgY. Interestingly, the TBA value reached the lowest (F(3, 176) = 1.529, p = 0.028) at 4,000 μg/ml IgY. The quality of broiler meat did not differ significantly between the groups fed 1,500 and 3,000 μg/ml IgY.
Table 8.
Impact of the different immunoglobulin Y (IgY) concentrations in the broilers' diet on meat quality.
| No. | Treatment | Nutritive value | TBA value (mg/kg malondialdehyde) | |||
|---|---|---|---|---|---|---|
| Protein (%) | Ash (%) | Moisture (%) | Fat (%) | |||
| 1 | Control | 18.31 ± 0.12 | 1.15 ± 0.05 | 78.10 ± 0.33 | 2.61 ± 0.01 | 0.29 ± 0.02 |
| 2 | 1,500 μg/ml IgY | 18.90 ± 0.11 | 1.21 ± 0.04 | 78.20 ± 0.45 | 2.32 ± 0.02 | 0.27 ± 0.02 |
| 3 | 3,000 μg/ml IgY | 20.20 ± 0.21 | 1.57 ± 0.06 | 75.30 ± 0.51 | 2.11 ± 0.04 | 0.25 ± 0.03 |
| 4 | 4,000 μg/ml IgY | 21.10 ± 0.27*** | 1.88 ± 0.05** | 73.10 ± 0.34* | 2.03 ± 0.05* | 0.23 ± 0.01* |
Data were presented as the mean ± SEM and analyzed using one-way ANOVA.
IgY, immunoglobulin Y; TBA, thiobarbituric acid; (TBA).
***p <0.001, **p <0.01, *p <0.05 (compared to the control group).
Discussion
The current study determined the optimal concentration of IgY in poultry ration as a potential agent to reduce the contamination level of E. coli in meat and intestinal content. The current results indicated that the activity display was increased; these changes in activity could be related to the involvement of IgY in bone health (31). IgY, at special concentrations as a feed additive, can induce many behavioral, physiological, immunological changes and also enhance meat quality. Previously, probiotics used immunomodulation to restore the host's behavior and health (32). However, only a few studies looked at the impact of IgY on chick behavior, performance, and carcass criteria. Our results indicated that IgY powder at 3,000 and 4,000 μg/ml significantly enhanced the percentages of scan feeding, drinking, and dust bathing in comparison to the control group and 1,500 μg/ml IgY. The greater feeding behavior at both 3,000 and 4,000 μg/ml IgY is a result of the positive effect of IgY on the intestinal microflora of birds and its microbiocide effect on pathogenic bacteria. Additionally, this increased the feed use and improved the poultry's health and their meat quality (33). The weights of the viscera and internal organs (heart and liver) were recorded in our previous article (12). Therefore, we confirmed that the IgY-supplemented diet improved the weights of carcasses and internal organs. Herein, we were interested to emphasize the impact of IgY on the digestive system-related organs such as crop, proventriculus, gizzard, and the intestine. Moreover, supplementing the diet with IgY powder at 3,000 and 4,000 μg/ml significantly enhanced the relative proventriculus and intestinal weights compared to those reared under diets of 0 and 1,500 μg/ml IgY. This result indicated that birds fed 3,000 and 4,000 μg/ml IgY had a slight improvement in their body weights.
Furthermore, the current results demonstrated that the drinking activity of chicks was significantly developed in the 3,000- and 4,000-μg/ml IgY groups in comparison to the control and 1,000 μg/ml IgY groups. The great water intake in birds may be attributed to the enhanced feeding activities (34). Also, the current results showed that IgY at different concentrations did not affect chick preening and head shaking activities in comparison to the control. However, broilers fed 3,000 and 4,000 μg/ml IgY had a significant increase in dust bathing and body stretching activities. This improvement in stretching movements may reflect the improvement of the health status of the birds (35).
Various approaches to reduce the microbial contamination of carcasses and meat products can improve the meat quality besides its shelf life (36). The high usage of drugs in the poultry industry leaves accumulating residual effects, and the genetic transfer associated with antibiotic resistance in meat is a serious concern threatening consumers of chicken meat (37). Therefore, natural ingredients in the poultry ration are extremely beneficial to poultry farms for improving public health and biosafety protocols. The expansion of IgY immunization is a strong alternative to antibiotics in poultry feed (38, 39). The antibacterial effect of IgY has been evaluated, according to data provided in vitro (40) and in animal health clinical studies (12, 23). The latest studies confirmed the influence of IgY as an active antibacterial agent for Gram-positive bacteria; however, no killing influence had been recorded for Gram-negative bacteria (12). Cytokine activity was highly stimulated by purified IgY compared to other food supplements (41). Moreover, in our study, broiler meat from the group supplemented with IgY showed the most desirable meat quality characteristics, color, shearing force, and water holding capacity (12). Therefore, feeding birds IgY during the production cycle can improve the conversion of their feed (7, 10, 25). We investigated the antimicrobial activity of multiple concentrations (1,500, 3,000, and 4,000 μg/ml) of IgY in vitro and discovered that 4,000 μg/ml IgY had bacteriocidal effect against E. coli. This finding supported the results of our previous report (12) indicating the therapeutic advantages of IgY against Gram-positive bacteria.
Our findings demonstrated that the mixing of IgY to the diet had little effect on FI until day 42, which agreed with previous reports (7, 12). However, the doses of 3,000 and 4,000 μg/ml IgY significantly improved the WG and FCR on days 21 and 42 compared to the control group. Concerning the impact of IgY on E. coli contamination, in the groups fed 3,000 and 4,000 μg/ml of IgY, the levels of E. coli in meat and cecal contents were significantly more reduced than in chickens that received the control diet. In addition, the performance and meat quality were improved at all three doses of IgY (1,500, 3,000, and 4,000 μg/ml) on days 21 and 42. In addition, the changes in the gastrointestinal tract microflora seemed to play a big role in lowering meat contamination (42). Hence, IgY, as the primary compound extracted from egg yolks, has proteolytic stability in the stomach and intestines if administered orally (43). Moreover, the stability of IgY in the alimentary tract is dependent on a number of factors involving the pH state and enzymatic activity (44). Consequently, it depends on the health conditions, feeding programs, and the age of chickens. Maternal antibodies are used as food additives for animals since they can proceed with the pelleting processes of the ration throughout the high temperature (45). The activity of an antibody may be improved when prepared at 95°C if particular carbohydrates are present in IgY preparations and/or steaming during the pellet processing to kill bacterial cells (26). While the heat stability of IgY is appropriate for mixing in the ration, we found that its binding activity dropped at extremely high temperatures (46).
In our study, 3,000 and 4,000 μg/ml of IgY led to a great WG and improvement of the FCR in chicken rations without any effects on the FI. Our results further demonstrated that adding 4,000 μg/ml IgY to the diet did influence the FI. The active ingredients in IgY play an essential role in the production and secretion of digestive enzymes (43). However, it requires further investigation whether the improved efficacy of the gastrointestinal tract resulting from IgY intake was linked to a lower intestinal bacterial load. Our results evidenced that birds exposed to IgY at a high concentration (4,000 μg/ml) had an improvement in the final BW and FCR due to high immunization. Such improvements in WG and immunity could be related to the capability of IgY to inhibit the proliferation of pathogenic bacteria and, therefore, improve feed utilization. In our work, the feed efficiency of broilers was not altered by adding 1,500 μg/ml IgY to the ration. The PCV values might change due to the immune response of broilers (25). It was reported that the PCV changed in the case of immune suppression (7, 47).
Herein, the PCV values, RBC counts, and Hb concentrations recorded non-significant differences, which confirmed that different concentrations of IgY supplementation showed no physiological changes. Moreover, the H/L ratio in the group fed 4,000 μg/ml IgY was relatively similar to the control. It revealed that the different concentrations of IgY supplementation failed to change the H/L ratio, which was supported by our previous results (7). However, in the group given 4,000 μg/ml IgY, the numbers of heterophils and eosinophils were lower. It could be caused by the failure of IgY to bind to Toll-like receptor 4 on immune cells, resulting in the cancellation of pro-inflammatory cytokines and the deactivation of heterophils (48). It was noticeable that RBCs, the Hb concentration, the percentage of PCV, and heterophils had the highest values when chickens were fed 1,500 μg/ml IgY but with no significant differences. Then, they returned relatively to normal values of the controls when the birds were fed a double concentration (3,000 μg/ml IgY). It seemed a type of compensatory mechanism that was essential for their static homeostasis. Moreover, we evidenced that the H/L ratio was altered in birds fed variable IgY concentrations due to individual physiological stress. However, there were no significant differences recorded for basophils, monocytes, and eosinophils. Therefore, the stability of the physiological parameters, as referred to above, would help in maintaining the passive immunization of the IgY supplement (49). Interestingly, the circulating IgY of the chicks can be determined by the levels in the plasma of the dam and passed on by the egg yolk (7, 10, 12, 50). The plasma IgY levels were lowered mostly on day 2, as it was expected up to 2,000 μg/ml, as previously indicated (51). Moreover, the IgY transfer from the mother to the chicks occurred on day 4 (1,010 μg/ml), followed by a significant decline on day 7 (830 μg/ml) (10, 28). The occurrence of the IgY half-life was in chick plasma on day 1, whereas it was reduced on days 2 and 3 (52), depending on the stored amount of IgY. Thus, the correlation between circulating IgY from the body of the chick and the level of their activities during their first week of life was strongly positive. Therefore, IgY plays a multidisciplinary role in the activation of early immunity and in chickens' health (53, 54). The disparity most likely occurred due to the wide variety of levels/concentrations of IgY in hens, which was consequently related to the withdrawal amount of IgY during the chick's aging process (51, 52).
The protein content and ash percentage were greater in the group fed 4,000 μg/ml IgY compared to the controls, demonstrating the improved broiler's meat quality in this target group. The higher stability of the fat was recorded in the group fed 4,000 μg/ml IgY due to the minimal TBA values, demonstrating that IgY plays an important role in enhancing the shelf life of broiler meat. Our results agreed with previous reports that discussed the role of the addition of probiotics to improve the TBA values during storage of broiler meat compared to the control group (55, 56).
The level of meat contamination with E. coli was lower, suggesting the enhanced hygienic quality of broiler carcasses in the group fed 3,000 and/or 4,000 μg/ml IgY. Using 1,500 μg/ml IgY supplementation was beneficial for improving the physiology of birds and their meat quality and had a bacteriocidal effect on Gram-positive, but not on Gram-negative bacteria. However, using higher concentrations such as 3,000 or 4,000 μg/ml IgY will be extremely beneficial for better meat quality and broad bacteriocidal effects on both Gram-positive and Gram-negative bacteria. We strongly recommend using 3,000 or 4,000 μg/ml IgY supplementation in the diet in large-scale production. These IgY concentrations played an integral antibacterial bioactivity role. Therefore, optimizing the concentration of IgY, a novel future of preventive medicine, reduces E. coli contamination and will keep the broilers, as well as consumers, healthy. Taken together, this novel dietary supplement will help achieve the concept of a One Health mission.
Conclusions
We conclude that supplementation of optimized concentrations of IgY in poultry rations improved the growth rate and the hygienic status of broiler meat. The obtained findings confirmed that the counts of E. coli in the meat and intestinal content were lowered by adding optimized concentrations of IgY (3,000 and/or 4,000 μg/ml) in poultry rations. Consequently, it reduced carcass contamination and improved the WG and FCR. Nevertheless, there was no significant impact on E. coli reduction after the addition of low concentrations of IgY to the broiler diet. Moreover, 1,500 μg/ml IgY improved the behavioral and physiological conditions of broilers, in addition to the enhanced nutritional benefits to consumers. We strongly recommend using IgY in large-scale poultry production due to its multidisciplinary advantages. Our work, therefore, has great potential in meeting the standard of the One Health assessment, especially regarding animal and human health.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics Statement
The animal study was reviewed and approved by all methodologies of animal testing were carried out in line with the Animal Care and Use Committee of Veterinary Medicine Faculty, Mansoura University, Mansoura, Egypt, Protocol Dated 20032020 gave its approval to the Animal Experimental Guidelines.
Author Contributions
IR and AA collaboratively shared for material preparations and the methodologies of this research. For the preparation of scientific paper, AR, MH, MM, and NE developed the hypothesis and concept of the study. GB, AE, MM, WE-G, SA-H, GE-N, BB, MA, MG, AH, and HG participated in the experimental methodologies and analysis, cooperated in the data analysis, writing, and revision of the manuscript. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors extend their gratitude to the Researchers Supporting Program (TUMA-Project-2021-6), Al Maarefa University, Riyadh, Saudi Arabia, for supporting the steps of this work. We acknowledge the program Biorender.com for the strategy design of the study. We thank the Faculty of Veterinary Medicine at Menofia and Mansoura Universities for assisting us in completing this project.
References
- 1.Das DJ, Shankar A, Johnson JB, Thomas S. Critical insights into antibiotic resistance transferability in probiotic Lactobacillus. Nutr. (2020) 69:110567. 10.1016/j.nut.2019.110567 [DOI] [PubMed] [Google Scholar]
- 2.Chung DM, Ferree E, Simon DM, Yeh PJ. Patterns of bird–bacteria associations. Ecohealth. (2018) 15:627–41. 10.1007/s10393-018-1342-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zulfakar SS, Abu-Bakar NF, Aidilputra AA, Miatong A, Chong ES. Microbial contamination of meat contact surfaces at the selected beef processing plants in selangor and its biofilm formation ability. Pertanika J Trop Agric Sc. (2019) 42: 709–726. 10.17221/65/2009-CJFS [DOI] [Google Scholar]
- 4.Raj H, Wiebe WJ, Liston J. Detection and enumeration of fecal indicator organisms in frozen sea foods: II. Enterococci Appl Environ Microbiol. (1961) 9:295–303. 10.1128/am.9.4.295-303.1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quinn PJ, Markey BK, Carter ME, Donnelly WJC, Leonard FC, Maguire D. Veterinary Microbiology and Microbial Diseases. 1st ed. MA: Blackwell; (2002). p. 1751–813. [Google Scholar]
- 6.Schade R, Chacana PA. Livetin fractions (IgY). In: Huopalahti R, López-Fandiño R, Anton M, Shade R, editors. Bioactive Egg Compounds. Springer: (2007). p.25–32. 10.1007/978-3-540-37885-3_5 [DOI] [Google Scholar]
- 7.Rehan IF, Youssef M, Abdel-Rahman MAM, Fahmy SG, Ahmed E, Ahmed AS, et al. The impact of probiotics and egg yolk IgY on behavior and blood parameters in a broiler immune stress model. Front Vet Sci. (2020) 7:145. 10.3389/fvets.2020.00145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gassmann M, Thömmes P, Weiser T. Hübscher U. Efficient production of chicken egg yolk antibodies against a conserved mammalian protein. The FASEB J. (1990) 4:2528–32. 10.1096/fasebj.4.8.1970792 [DOI] [PubMed] [Google Scholar]
- 9.Batty I. The transmission of passive immunity from mother to young. Immunology. (1971) 20:119.5320984 [Google Scholar]
- 10.Rehan IF, Mohammed HH, Fahmy SG, Elnagar A, Youssef M, Shanab O. Influence of photoperiod and circulating-IgY on some behavioural patterns of chicks during the first week of life. J Vet Sci Anim Husb. (2019) 4:18–25. Available online at: https://www.veterinarypaper.com/pdf/2019/vol4issue2/PartA/4-2-2-815.pdf [Google Scholar]
- 11.Ferreira Júnior Á, Santos JPD, Sousa IDO, Martin I, Alves EGL, Rosado IR. Gallus gallus domesticus: immune system and its potential for generation of immunobiologics. Ciência Rural, Santa Maria. (2018) 48:e20180250. 10.1590/0103-8478cr20180250 [DOI] [Google Scholar]
- 12.Hussein MA, Rehan IF, Rehan AF, Eleiwa NZ, Abdel-Rahman MA, Fahmy SG, et al. Egg yolk IgY: a novel trend of feed additives to limit drugs and to improve poultry meat quality. Front Vet Sci. (2020) 7:350. 10.3389/fvets.2020.00350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Research Council (NRC) . Nutrient Requirements of Poultry. 9th ed. Washington, DC: National Academy Press; (1994). p. 0377–8401. [Google Scholar]
- 14.Williams T, Athrey G. Cloacal swabs are unreliable sources for estimating lower gastro-intestinal tract microbiota membership and structure in broiler chickens. Microorganisms. (2020) 8:718. 10.3390/microorganisms8050718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amro WA, Al-Qaisi W, Al-Razem F. Production and purification of IgY antibodies from chicken egg yolk. J Gen Eng, and Biot. (2018) 16:99–103. 10.1016/j.jgeb.2017.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Meulenaer B, Huyghebaert A. Isolation and purification of chicken egg yolk immunoglobulins: a review. Food Agri Immunol. (2001) 13: 275–88. 10.1080/09540100120094537 [DOI] [Google Scholar]
- 17.Arnould C, Faure JM. Use of pen space and activity of broiler chickens reared at two different densities. Appl Anim Behav Sci. (2004) 87:155–70. 10.1016/j.applanim.2004.01.001 [DOI] [Google Scholar]
- 18.Benjamin MM. Outline of Veterinary Clinical Pathology. 3rd ed. Ames, IA: The Iowa State University Press; (1979). [Google Scholar]
- 19.Franco RS. Hemoglobin. In: Kaplan LA, Pesce AJ, Kazmierczak SC, editors. Clinical chemistry. St Louis, MO; Toronto, ON; Princeton, NJ: The CV Mosby Co. (1984) 1294–1296. [Google Scholar]
- 20.He SP, Arowolo MA, Medrano RF Li S, Yu QF, Chen JY, et al. Impact of heat stress and nutritional interventions on poultry production. World Poult Sci J. (2018) 74:647–64. 10.1017/S004393391800072733025116 [DOI] [Google Scholar]
- 21.Ibrahim HR, Thomas U, Pellegrini A. A helix-loop-helix peptide at the upper lip of the active site cleft of lysozyme confers potent antimicrobial activity with membrane permeabilization action. J Biol Chem. (2001) 276:43767–74. 10.1074/jbc.M106317200 [DOI] [PubMed] [Google Scholar]
- 22.Ibrahim HR, Inazaki D, Abdou A, Aoki T, Kim M. Processing of lysozyme at distinct loops by pepsin: a novel action for generating multiple antimicrobial peptide motifs in the newborn stomach. Biochim Biophys Acta. (2005) 1726:102–14. 10.1016/j.bbagen.2005.07.008 [DOI] [PubMed] [Google Scholar]
- 23.Diraviyam T, Ambi SV, Vieira-Pires RS, Xiaoying Z, Sekaran S, Krishnan U. Chicken egg yolk antibody (IgY) as diagnostics and therapeutics in parasitic infections—a review. Int. J. Biol. Macromol. (2019) 1: 755–63. 10.1016/j.ijbiomac.2019.06.118 [DOI] [PubMed] [Google Scholar]
- 24.Hoq M, Mitsuno K, Tsujino Y, Aoki T, Ibrahim H. Triclosan–lysozyme complex as novel antimicrobial macromolecule: a new potential of lysozyme as phenolic drug-targeting molecule. Int J Biol Macromol. (2008) 42:468–77. 10.1016/j.ijbiomac.2008.03.003 [DOI] [PubMed] [Google Scholar]
- 25.Goldstein EJ, Tyrrell KL, Citron DM. Lactobacillus species: taxonomic complexity and controversial susceptibilities. Clin Infect Dis. (2015) 60:98–107. 10.1093/cid/civ072 [DOI] [PubMed] [Google Scholar]
- 26.Bobeck EA, Cook CL, Gelbach BE, Yang M, Cook ME. Thermoprotection of bioactive proteins added to animal feed. World Poultry Sci J. (2008) 64:199. Available online at: https://nanopdf.com/download/elizabeth-bobeck-kraayenbrink-phd_pdf [Google Scholar]
- 27.AOAC. Official Methods of Analysis of the AOAC International: AOAC International 17th ed. Gaithersburg, MD: The Association of Official Analytical Chemists (2000). Available online at: https://www.scirp.org/(S(351jmbntvnsjt1aadkposzje))/reference/ReferencesPapers.aspx?ReferenceID=1687699
- 28.Arnold KB, Chung AW. Prospects from systems serology research. Immunol. (2018) 153:279–89. 10.1111/imm.12861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vouga M, Greub G. Emerging bacterial pathogens: the past and beyond. Clin Microbiol Infect. (2016) 22:12–21. 10.1016/j.cmi.2015.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.SPSS Inc. Released SPSS for Windows, Version 16.0. Chicago, IL: SPSS Inc; (2007). [Google Scholar]
- 31.Wang WC, Yan FF, Hu JY, Amen OA. Cheng HW. Supplementation of Bacillus subtilis-based probiotic reduces heat stress-related behaviors and inflammatory response in broiler chickens. J Anim Sci. (2018) 96:1654–66. 10.1093/jas/sky092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goto H, Sagitani A, Ashida N, Kato S, Hirota T, Shinoda T., et al. Anti-influenza virus effects of both live and non-live Lactobacillus acidophilus L-92 accompanied by the activation of innate immunity. Br J Nutr. (2013) 110:1810–8. 10.1017/S0007114513001104 [DOI] [PubMed] [Google Scholar]
- 33.Kota RK, Reddy PN, Sreerama K. Application of IgY antibodies against staphylococcal protein A (SpA) of Staphylococcus aureus for detection and prophylactic functions. Appl Microbiol Biotech. (2020) 104: 9387–98. 10.1007/s00253-020-10912-5 [DOI] [PubMed] [Google Scholar]
- 34.Feddes JJ, Emmanuel EJ, Zuidhoft MJ. Broiler performance, body weight variance, feed and water intake, and carcass quality at different stocking densities. Poult Sci. (2002) 81:774–9. 10.1093/ps/81.6.774 [DOI] [PubMed] [Google Scholar]
- 35.Mahmoud UT, Elsayed MS. Behavioral and physiological effects of mannan-oligosaccharide and β-glucan prebiotic combination on heat stressed broiler chickens. J Adv Vet Anim Res. (2017) 7:81–6. Available online at: https://www.advetresearch.com/index.php/AVR/article/view/258 [Google Scholar]
- 36.Piatek J, Sommermeyer H, Bernatek M, Ciechelska-Rybarczyk A, Oleskow B, Mikkelsen LS, et al. Persistent infection by salmonellaentericaservovartyphimurium: are synbiotics a therapeutic option?–A case report. Benef Microbes. (2019) 10:211–7. 10.3920/BM2018.0080 [DOI] [PubMed] [Google Scholar]
- 37.Al-Nasser AY, Al-Zenki SF, Al-Saffar AE, Abdullah FK, Al-Bahouh ME, Mashaly M. Zeolite as a feed additive to reduce Salmonella and improve production performance in broilers. Int J Poult Sci. (2011) 10:448–54. 10.3923/ijps.2011.448.454 [DOI] [Google Scholar]
- 38.Michael A, Meenatchisundaram S, Parameswari G, Subbraj T, Selvakumaran R, Ramalingam S. Chicken egg yolk antibodies (IgY) as an alternative to mammalian antibodies. Indian J Sci Technol. (2010) 3:468–74. 10.17485/ijst/2010/v3i4.24 [DOI] [Google Scholar]
- 39.Munhoz LS, Vargas GD, Fischer G, Lima MD, Esteves PA, Hübner SD. Avian IgY antibodies: characteristics and applications in immunodiagnostic. Ciência Rural. (2014) 44:153–60. 10.1590/S0103-84782014000100025 [DOI] [Google Scholar]
- 40.Gadde U, Rathinam T, Lillehoj HS. Passive immunization with hyperimmune egg-yolk IgY as prophylaxis and therapy for poultry diseases – a review. Anim Health Res Rev. (2015) 16:163–76. 10.1017/S1466252315000195 [DOI] [PubMed] [Google Scholar]
- 41.Bizanov G. IgY extraction and purification from chicken egg yolk. J Hellenic Vet Med Soc. (2017) 68:265–72. 10.12681/jhvms.15466 [DOI] [Google Scholar]
- 42.Açikgöz Z, Bayraktar H, Altan ÖZ. Effects of formic acid administration in the drinking water on performance, intestinal microflora and carcass contamination in male broilers under high ambient temperature. Asian-Australasian Anim Sci J. (2010) 24:96–102. 10.5713/ajas.2011.10195 [DOI] [Google Scholar]
- 43.Hatta H, Tsuda K, Akachi S, Kim M, Yamamoto T, Ebina T. Oral passive immunization effect of anti-human rotavirus IgY and its behavior against proteolytic enzymes. Biosc Biotech and biochem. (1993) 57:1077–81. 10.1271/bbb.57.1077 [DOI] [PubMed] [Google Scholar]
- 44.Akita EM, Nakai S. Production and purification of Fab' fragments from chicken egg yolk immunoglobulin Y (IgY). J Immunol methods. (1993) 162:155–64. 10.1016/0022-1759(93)90380-P [DOI] [PubMed] [Google Scholar]
- 45.Schade R, Zhang XY, Terzolo HR. Use of IgY antibodies in human and veterinary medicine. in Bioactive Egg Compounds. Berlin (2007). p. 213–22.16180988 [Google Scholar]
- 46.Jaradat ZW, Marquardt RR. Studies on the stability of chicken IgY in different sugars, complex carbohydrates and food materials. Food and Agric Immunol. (2000) 12:263–72. 10.1080/09540100020008137 [DOI] [Google Scholar]
- 47.Xie H, Rath NC, Huff GR, Huff WE, Balog JM. Effects of Salmonella typhimurium lipopolysaccharide on broiler chickens. Poult Sci. (2000) 79:33–40. 10.1093/ps/79.1.33 [DOI] [PubMed] [Google Scholar]
- 48.Rohde F, Schusser B, Hron T, Farkašová H, Plachý J, Härtle S, et al. Characterization of chicken tumor necrosis factor-α, a long missed cytokine in birds. Front Immunol. (2018) 9:605. 10.3389/fimmu.2018.00605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abou Alazab MF, Horiuchi H, Furusawa S. Induction of non-specific suppression in chicks by specific combination of maternal antibody and related antigen. J Vet Med Sci. (2015) 77:1363–9. 10.1292/jvms.14-0525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mukherjee S, Karmakar S, Babu SPS. TLR2 and TLR4 mediated host immune responses in major infectious diseases: a review. Braz J Infect Dis. (2016) 20: 193–204. 10.1016/j.bjid.2015.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kowalczyk K, Daiss J, Halpern J, Roth TF. Quantitation of maternal-fetal IgG transport in the chicken. Immunol. (1985) 54:755–62. [PMC free article] [PubMed] [Google Scholar]
- 52.Al-Natour MQ, Ward LA, Saif YM, Stewart B, Keck LD. Effect of different levels of maternally derived antibodies on protection against infectious bursal disease virus. Avian Dis. (2004) 48:177–82. 10.1637/5319 [DOI] [PubMed] [Google Scholar]
- 53.Cook ME, Trott DL. IgY–Immune component of eggs as a source of passive immunity for animals and humans. World's Poult Sci J. (2010) 66:215–26. 10.1017/S0043933910000279 [DOI] [Google Scholar]
- 54.Kovacs-Nolan J, Mine Y. Egg yolk antibodies for passive immunity. Ann review of Food Sci and technol. (2012) 3:163–82. 10.1146/annurev-food-022811-101137 [DOI] [PubMed] [Google Scholar]
- 55.Pelicano ERL, Souza PA, De Souza HBE, Oba A, Norkus EA, Kodawara LM, et al. Effect of different probiotics on broiler carcass and meat quality. Rev Bras Ciência Avícola. (2003) 5:207–14. 10.1590/S1516-635X2003000300009 [DOI] [Google Scholar]
- 56.Ali FHM. “Probiotics feed supplement” to improve quality of broiler chicken carcasses. World J Dairy Food Sci. (2010) 5:93–99. Available online at: https://www.cabdirect.org/cabdirect/abstract/20103317627 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.