Abstract
Oral drug delivery systems (ODDSs) have various advantages of simple operation and few side effects. ODDSs are highly desirable for colon-targeted therapy (e.g. ulcerative colitis and colorectal cancer), as they improve therapeutic efficiency and reduce systemic toxicity. Chitosan/alginate nanoparticles (CANPs) show strong electrostatic interaction between the carboxyl group of alginates and the amino group of chitosan which leads to shrinkage and gel formation at low pH, thereby protecting the drugs from the gastrointestinal tract (GIT) and aggressive gastric environment. Meanwhile, CANPs as biocompatible polymer, show intestinal mucosal adhesion, which could extend the retention time of drugs on inflammatory sites. Recently, CANPs have attracted increasing interest as colon-targeted oral drug delivery system for intestinal diseases. The purpose of this review is to summarize the application and treatment of CANPs in intestinal diseases and insulin delivery. And then provide a future perspective of the potential and development direction of CANPs as colon-targeted ODDSs.
Keywords: Oral drug delivery, chitosan/alginate nanoparticle, insulin, ulcerative colitis, colon cancer
Introduction
Oral drug delivery systems (ODDSs) are effective strategy for intestinal disease treatment due to its convenient, patient-friendly, painless, and noninvasive properties (Renukuntla et al., 2013). Despite possessing numerous advantages, ODDSs are susceptible to absorption denaturation and degradation by the gastrointestinal tract (GIT) barriers, resulting in malabsorption and reduced effectiveness (even ineffective) at the disease site (Dos Santos et al., 2021). Meanwhile, protein and peptide drugs in ODDSs administration are susceptible to denaturation and degradation by digestive enzymes in the GIT (Haddadzadegan et al., 2022).
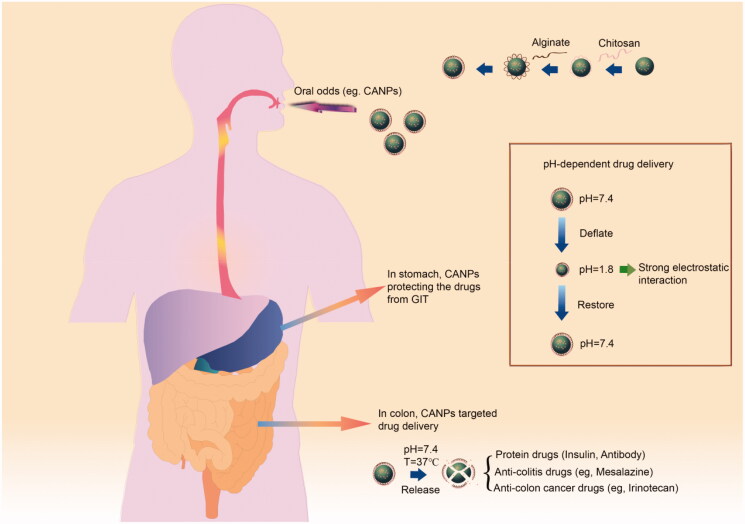
In general, the GIT is divided into three major regions: the stomach (pH 1.5–3.5), the small intestine (pH 5.5–6.8) and the large intestine (pH 7.0–8.0) (Chambin et al., 2006). Generally, oral colon-targeted drug delivery systems are pH-dependent and designed based on progressively increasing pH gradient in the GIT (Figure 1) (Keppler and Humpf, 2005). Typical pH-dependent nanomaterials include acrylate copolymers (eudragit), o-phenylene dimethyl acetate cellulose, chitosan, pectin, polylactic acid-hydroxyacetic acid copolymer (PLGA), etc. Among them, the polysaccharide-based materials offer significant advantages in terms of biodegradability, biocompatibility, adhesion, safety, and gelation properties (Yu et al., 2019).
Figure 1.
CANPs and its oral pH-dependent drug delivery property.
Among those polysaccharides, marine derived polysaccharides have various advantages of being widely distributed, abundant content, simple to manufacture, biodegradable and biocompatible (Manivasagan et al., 2016; Chen et al., 2020). Chitosan and alginate as abundant and versatile marine polysaccharides, showed excellent mucosal adhesion, biodegradability and biocompatibility (Bhunchu and Rojsitthisak, 2014; Chu and Wang, 2020; Sudhakar et al., 2020; Taemeh et al., 2020). Chitosan/alginate nanoparticles (CANPs), prepared by the ionic gel method using chitosan and alginate as shells, have attracted attention as oral drug carriers (Hagras et al., 2022; Li et al., 2022). CANPs exhibited strong electrostatic interactions between carboxyl groups of alginate and amino groups of chitosan, resulting in shrinkage and gel formation at low pH, thereby protecting the drugs, from GIT and the aggressive gastric environment. Moreover, oral CANPs used for colon-targeted drug delivery have certain advantages, including increased drug absorption, improved oral drug bioavailability and therapeutic efficiency, reduced systemic toxicity and reduced administered dosage (Kumar and Mishra, 2008). These properties make CANPs an emerging and promising ODDSs.
The purpose of this review is to introduce the preparation methods and related properties of CANPs, and summarize their recent application on insulin delivery and intestinal diseases, e.g. colorectal cancer (CRC) and ulcerative colitis (UC). Furthermore, we also discussed the prospects and challenges of CANPs.
Preparation methods and properties of CANPs
Ionic gel is the most commonly used methods for preparing CANPs, which was first proposed by Calvo et al. (Calvo et al., 1997). The mechanism of ionic gel method is based on electrostatic interactions between chitosan and alginate. Concretely, the physical interactions are from the negatively charged carboxylic acid group of alginate and the positively charged amino group of chitosan, which leads to shrinkage and gel formation at low pH, thereby protecting the drugs from GIT and the aggressive gastric environment (Lanjhiyana et al., 2013; Shinde et al., 2014). This method is generally carried out by dropping the drug-laden core polymer into an aqueous solution of polyvalent cations, followed by the addition of the polyanionic or anionic polymer under mechanical stirring at room temperature (Naskar et al., 2019; Lang et al., 2020).
Ionic gel method for CANPs has many advantages, such as the protocol are simple and fast, and easy to reach high encapsulation rate. Nevertheless, this method does not allow for a narrow particle size distribution, which will indirectly affect the drug loading capacity (Li et al., 2019).There are also other methods for CANPs preparation, including emulsification (Mao et al., 2016; Kyzioł et al., 2017; Khorshidian et al., 2019; Yue et al., 2020), electrostatic gelation (Aluani et al., 2017; Yoncheva et al., 2021), extrusion coagulation (ALQuadeib et al., 2020), co-precipitation (Yu et al., 2019), etcs. These methods are more complex than ionic gel method, while the control of particle size and distribution are more rigorous.
The properties of CANPs meet the criteria for an oral drug carrier. (1) CANPs protect the drugs in the GIT. CANPs as pH-dependent drug carriers could shrink at low pH through strong electrostatic interaction, thus protecting the drug from the aggressive gastric environment (Chen et al., 2019). (2) The size, shape and distribution of CANPs meet the requirements of ODDS. Significantly, the size of CANPs suitable for oral supplement in previous studies commonly ranges from 100-900 nm, which always exhibited higher efficiency of cellular internalization, stronger in vitro anti-inflammatory ability and better therapeutic effects. (3) CANPs are characterized by high drug loading capacity and high encapsulation efficiency. The method of preparation and the physicochemical properties of the drug determine the drug loading capacity and encapsulation rate of the carrier. (4) CANPs have suitable-sustained release properties. CANPs have better sustained release properties than naked drugs due to their pH-dependent nature, resulting in prolonged inflammatory or tumor suppression. (5) CANPs are nontoxic and degradable drug carriers. Alginate is a water-soluble, naturally occurring linear polysaccharide, and this biopolymer is nontoxic, biocompatible, biodegradable, with low immunogenicity and good mucosal adhesion, facilitating its use in oral drug delivery (Mukhopadhyay et al., 2015). Similarly, chitosan is biocompatible, biodegradable, nontoxic and non-immunogenic polymer with significant adsorption and antifungal activity, making it a great potential for use in food and pharmaceutical applications. Overall, CANPs have a wide range of applications as carriers for oral drug delivery (Venkatesan et al., 2017).
Application of CANPs in colitis therapy
UC is a chronic disease that is increasing in incidence and prevalence every year (Segal et al., 2021) and is characterized by a long, incurable and recurrent course, with clinical manifestations such as bloody diarrhea, frequency, abdominal pain, weakness and fecal incontinence (Kobayashi et al., 2020). Oral drugs for UC treatment, such as 5-aminosalicylic acid (5-ASA) and glucocorticoid hormones (GCS), are rapidly and widely absorbed in the upper GIT, and may cause a range of side effects such as diarrhea, nausea, abdominal pain, headache, vomiting, and rash (Nunthanid et al., 2008). CANPs, as an emerging and promising ODDSs, have been reported for the treatment of UC (Table 1).
Table 1.
Application of chitosan/alginate nanocarriers in the treatment of colitis.
| Innovation points | Advantage | Particle size (nm) | Content (%) | Encapsulation efficiency (%) | Ref |
|---|---|---|---|---|---|
| PH-dependent release of curcumin from CAP1AG4 CH5@CUNCs | Enhanced accumulation in the inflamed colonic tissues | 421 ± 14 | 20–80 | 90 | (Oshi et al., 2020) |
| CD-Cur-CANPs with pH-sensitive and α-amylase-responsive release character-istics | Strong colonic biodistribution and accumulation, rapid macrophage uptake, promoted colonic epithelial barrier integrity and modulated production of inflammatory cytokines | 462.1 | 3.49 | 88.89 | (Li et al., 2021) |
| A pH-sensitive hydrogel formed by chitosan and sodium alginate under the mechanochemical force | 5-ASA colon-targeted delivery system without a crosslinking agent | 489.57 ± 118.07 | 3.77 | – | (Xu et al., 2021) |
| Novel pH-sensitive hydrogel beads based on gelatin/sodium alginate/ chitosan loaded with propolis ethanolic extracts | Synthesis of pH-sensitive hydrogel beads for different propolis ethanolic extracts | 106 −2 × 106 | – | – | (Ceylan et al., 2021) |
| A series of curcumin loaded polymeric nanoparticle (NPs) with different particle sizes | The surface functionalization of PF127 can enable NPs to penetrate through the mucus layer Improve the cellular uptake efficiency of NPs by macrophages |
185–884 | 5.1–6.1 | 73.2–89.6 | (Zhou et al., 2020) |
Oshi et al. developed orally administered core-shell nanoparticles with a core of curcumin nanocrystals (CUNCs) and a shell layer of CANPs to successfully reduce symptoms associated with inflammation in dextran sodium sulfate (DSS)-induced colitis mice model (Oshi et al., 2020). Through this ODDS, they aimed to precisely deliver CUNCs to the colon and protect the drugs from GIT, resulting in a significantly therapeutic effect. During UC treatment, pH-triggered surface charge inversion of CANPs promotes the adhesion and accumulation of CUNCs in inflamed colonic tissue by causing them to interact with negatively charged mucins in the mucosa.
In our previous study, an effective enzyme-triggered controlled release system using a curcumin-cyclodextrin (CD-Cur) inclusion as the core and CANPs as the shell (Li et al., 2021). This specific colon-targeted drug delivery system showed significant pH-dependent properties, protecting the drugs through GIT. Meanwhile, taking advantage of the characteristics of α-amylase produced by gut microbiota to specifically hydrolyze the CD-Cur core, this enzyme-triggered controlled release system showed promising therapeutic effects on DSS-induced colitis in mice, well protected the colon, promoted the integrity of the colonic epithelial barrier and remodeled the intestinal microbiota.
In order to overcome the problems of complex preparation process, poor biocompatibility and delayed drug release of current oral targeted drug delivery systems, Xu et al. prepared 5-ASA-loaded colon-targeted hydrogels without non-crosslinkers in a single step by applying pH-sensitive characteristics through a mechanochemical approach (Xu et al., 2021). Experimental studies have shown that this colon-targeted hydrogel has a release rate of 28.19% in simulated gastric fluid, which is 62.83% lower than the 5-ASA active pharmaceutical ingredient (API), while the release rate in simulated colonic fluid is similar to that of the 5-ASA API. It can be seen that colon-targeted drug delivery systems are simple to prepare, and can improve the therapeutic effect of drugs by targeting the lesion sites in the colon.
Propolis is a resinous substance with anti-inflammatory, antibacterial, and antioxidant properties, and ethanolic extracts are the main form of propolis used in the treatment of colitis. Ceylan et al. synthesized a novel pH-sensitive hydrogel bead based on gelatin/sodium alginate/chitosan (GEL/SA/CS) loaded with propolis ethanol extract (PE) (Ceylan et al., 2021). Propolis was distributed in the carrier matrix by using GEL in the formulation and the outer surface of the carrier beads was covered with CS to increase the antibacterial properties, as well as to control the rate of propolis release. These propolis hydrogel microbeads have been shown to increase antibacterial activity in gastrointestinal environments at different pH levels and over different time periods, better targeting colitis treatment.
Recent studies have indicated that particle size is a critical factor influencing the phagocytic response of macrophages (Anderson et al., 2015; Jo et al., 2015; Jindal, 2017). To elucidate the effect of particle size on the cellular internalization and anti-inflammatory activity of orally administered nanomedicines, Zhou et al. prepared CuR-loaded nanoparticles with hydrodynamic particle sizes of 185, 474 and 883 nm, named NPs (200), NPs (500) and NPs (900), respectively, using a single-emulsion solvent volatilization method (Zhou et al., 2020). In vitro cellular uptake experiments showed that NPs (900) had the highest phagocytic efficiency on macrophages, while in vivo experiments demonstrated that NPs (900) had better efficacy. It follows that, within a certain size range, larger nanoparticles seem to respond better to phagocytosis by macrophages and have better efficacy.
Several of the nanocarriers mentioned above have been optimized with different treatments so that the drugs can be more accurately targeted to the inflammatory sites of colitis to work. In summary, nanoparticle systems have been identified as promising strategies for colon-targeted drug delivery purposes (Naeem et al., 2020). On the other hand, we propose that when treating UC via nanocarriers, consideration should be given to including physiological or pathological conditions related to cells/tissues damaged, loss of barrier function, mucus damage and changes in microbiota homeostasis, based on which to design more complex drug delivery system.
Application of CANPs in Colon cancer treatment
CRC is the third most common malignancy and the fourth leading cause of cancer-related death in the world. Until now, surgical resection is the most common treatment for CRC, but it is usually accompanied by the removal of a portion of a healthy colon or rectum and nearby lymph nodes. Even worse, a large number of CRC patients have common complications during surgery and often develop recurrence, which makes CRC difficult to cure. Common chemotherapeutic drugs used to treat CRC are 5-fluorouracil (5-FU), oxaliplatin, and methotrexate, which are usually administered intravenously, exposing healthy tissues to toxic chemotherapeutic drugs, which limit the dose and effectiveness of the drugs and have more side effects. CANPs, as a colon-targeted drug delivery system, have become a potential CRC drug nanocarrier (Table 2).
Table 2.
Application of chitosan/alginate nanocarriers in the treatment of colon cancer.
| Innovation points | Advantage | Particle size (μm) | Content (%) | Encapsulation efficiency (%) | Ref |
|---|---|---|---|---|---|
| Alginate and alginate-chitosan beads containing celecoxib solubilized into a self-emulsifying phase | Delay the drug release in acidic environment and to promote it in the intestinal compartment | 715–896 | 39.78–49.63 | – | (Segale et al., 2016) |
| Efficacy of the novel biopolymeric complex multiparticulate system consisting of chitosan, succinate and alginate for the capecitabine | CS-SA beads prolong the release of capecitabine in the colonic region, and also enhance antitumor efficacy | 846.21 ± 5.46 | 98.26 ± 3.14 | 85.63 ± 2.03 | (Sinha et al., 2018) |
| 5-Fluorouracil (5-FU) loaded layer-by-layer (LBL) film prepared by sequential adsorption of chitosan and alginate polyelectrolytes | The drug payload could be increased by preparing films in a LBL self-assembled manner | 145.0 ± 11.6 | 1837.2 ± 119.9 (μg/cm2 area of film) | – | (Janardhanam et al., 2020) |
| Optimized chitosan-Ca-alginate beads loaded with acid-resistant particles of 5-FU | Prolong residence time in colon, control release of encapsulated drug | 14.74 ± 0.09 | 49.01 ± 1.8 (mg/g MP) | 72.78 ± 1.10 | (Glavas Dodov et al., 2009) |
| Crosslinked polycation MPs loaded with acid-resistant particles of 5-FU and functionalized with wheat germ agglutinin (WGA) | Deliver the drug molecules to colon region, affect the transport of 5-FU into the cells | 14.7 | 82–90 | 72.8 | (Glavas-Dodov et al., 2013) |
| Sodium alginate as the shell layer and the quercetin-loaded chitosan nanoparticles and prebiotics as the core layer | Prebiotic activity and an enhanced colon cancer prevention property | 188.3 (nm) | 11.53 | 92.2 | (Wen et al., 2018) |
| Innovation points: Alginate-coupled trimethyl chitosan (TMC) loaded anti-gp130 and anti-S1PR1 siRNAs | Immunotherapy of cancer by specific silencing of tumor target antigens by NPs loaded with small interfering RNA (siRNA) | 110 (nm) | 30 | – | (Rostami et al., 2020) |
Celecoxib is a non-steroidal anti-inflammatory drug which has significant anti-cancer activity against CRC. Lorena Segale et al. used CANPs to prepare celecoxib microspheres which can minimize the release of celecoxib in the acidic environment and enhance its release in the intestinal lumen (Segale et al., 2016). Furthermore, they demonstrated that CANPs beads could ensure colonic administration of celecoxib by reducing the drug release rate under neutral pH conditions. Sinha et al. developed a novel biopolymeric complex multiarticulate system consisting of chitosan succinate and alginate (CS-SA) to encase capecitabine for CRC therapy. First, they optimized CS-SA using a Box-Behnken design according to three considerations of encapsulation efficiency, size, and release efficiency. The experiment data showed that the optimized CS-SA swelled the most in the intestinal environment of pH 7.4, while hardly swelled in the acidic environment, thus achieving the effect of protecting the drug and effectively acting on the colon (Sinha et al., 2018).
Layer-by-layer (LBL) self-assembled methods was also used to prepare CANPs for oral CRC treatment (Janardhanam et al., 2020). This LBL self-assembly method prepares films that greatly improve the high loading efficiency of 5-FU by using polycaprolactone (PCL, 95% w/w) as the backing. In addition, selective tumor lesion binding and targeting capabilities were obtained by functionalizing the outer-most layer with folic acid. It was found that the 5-FU LBL film not only had higher stability, but also exhibited a stronger cytotoxic effect on colon cancer cell lines than the 5-FU solution preparation.
Furthermore, to improve the oral administration ability of 5-FU in CANPs, Dodov et al. prepared lectin-coupled chitosan-Ca-alginate microparticles (MPs) (Glavas-Dodov et al., 2013). The lectin used herein was wheat germ agglutinin (WGA, 36 kDa), one of the least immunogenic lectins that has been shown to be more specific to human intestinal cell lines and human colon cells (Glavas Dodov et al., 2009). The experimental results showed that MPs loaded with 5-FU acid-resistant particles could deliver 5-FU to the colon area after being functionalized by WGA, and had good interaction with the colonic mucosa surface and could prolong the action time in the colon area.
Quercetin is a natural flavonoid compound that has the effect of preventing colon cancer, however, poor stability and low oral bioavailability limit its use. Wen et al. prepare quercetin loaded CANPs, using sodium alginate as shell, quercetin-chitosan nanoparticle and as the core layer. Then, they prepared a quercetin-loaded electrospunfiber mat (Q-EFM) containing prebiotics (GOS) (Wen et al., 2018). The experimental results showed that quercetin could be sustained and targeted released in the colon by Q-EFM, and the presence of GOS could increase the quercetin release rate. Therefore, this Q-EFM with prebiotic function can be used as a new type of colon-targeted delivery system to enhance anti-CRC effect.
An interconnected network between S1P/phosphosynuclein-1-phosphate receptor 1 (S1PR1), IL-6/glycoprotein 130 (GP130), and signal transducer and activator of transcription 3 (STAT3) signaling pathways, promotes cancer progression. Therefore, Rostami et al. used alginate coupled trimethyl chitosan (ATMC) NPs loaded with small interfering RNA (siRNA) to silence STAT3 upstream targets, including S1PR1 and GP130, finding that the siRNA-loaded NPs successfully inhibited the colony formation and migration of cancer cells (Rostami et al., 2020).
An important issue for drug delivery systems for the treatment of CRC is that the tumor-forming microenvironment leads to tissue hypoxia, which releases a protein called hypoxia-inducible transcription factor (You et al., 2016), the abnormal release of this protein will ultimately lead to vascular damage, and the damaged blood vessels will then hinder the delivery of essential nutrients to the diseased tissue, directly affecting the absorption of drugs, which puts forward higher requirements for the efficiency of the drug delivery system. Furthermore, drug delivery must pass through a kinetic barrier from blood flow to intracellular transport, which may be compromised in tumor tissue due to the heterogeneous structure of the tumor vessels, thus impairing the homogeneity of drug distribution (Kato et al., 2013; Suarato et al., 2016). Therefore, when designing colon-targeted drug delivery systems, the above-mentioned problems associated with CRC should be fully considered, especially the physiological and pathological characteristics associated with CRC.
The application of CANPs in oral insulin delivery
Diabetes is one of the most common chronic metabolic diseases in the world. Repeated subcutaneous injections of insulin disturbs the lives of patients with insulin-dependent diabetes. Oral administration of insulin is patient-friendly and cost-effective. As a protein drug, the orally administered insulin must pass through many physiological barriers, such as GIT, mucus layer, intestinal epithelium, then finally reach the circulatory system (Chen et al., 2011). Thus far, oral absorption of insulin remains a major scientific challenge. First, oral insulin must be effectively transported along the GIT tract without being degraded by the acidic conditions in the stomach and proteases in the gastrointestinal tract. Secondly, insulin is a hydrophilic protein, which is difficult to be encapsulated in a hydrophobic macromolecular carrier. Third, the bioavailability of untreated insulin is extremely low due to the first-pass effect in the liver. Thus far, many researchers have used CANPs as the carrier of insulin delivery (Table 3).
Table 3.
Innovation of chitosan/alginate nanoparticles in the treatment of diabetes.
| Innovation points | Advantage | Particle size (nm) | Content (%) | Encapsulation efficiency (%) | Ref |
|---|---|---|---|---|---|
| Calcium ions were added into chitosan/ alginate nanoparticles to form microspheres | Protect insulin | 194.25 ± 51.25 | 11.45 | 23.70 | (Li et al., 2021) |
| Stearic acid and alginate form alginate stearic acid nanopartic-les (ASAN), then cross-linked with oleic acid modified chitosan | Improve the encapsulation of insulin | 618.87 ± 6.57 | 6.44 | 76.69 | (Alfatama et al., 2018) |
| Alginate combined with C-18 to form alginate-c18 conjugate nanoparticles (AC18N), then cross-linked with oleic acid modified chitosan | Reduce its toxicity and enhance mucus penetration and intracellular transport | 522.50 ± 66.47 | 3.77 | 44.87 | (Alfatama et al., 2018) |
| Polyalacturonic acid (PGLA), chitosan, and alginate NPs | Avoid intestinal degradation caused by pH sensitivity and improves the overall blood sugar lowering effect | 225 ± 75 | 34.13 | 35.56 | (Zhang et al., 2018) |
| Bichitosan/albumin coated alginate /dextran sulfate nanoparticles | Improve mucosal adhesion efficiency and insulin permeability | 313.2 ± 2.8 | 10.10 | 72.40 | (Lopes et al., 2016) |
| Calcium phosphate is the core of nanoparticles, Vitamin B12 grafted Chitosan and sodium alginate used as cationic and anionic polyelectrolyte, respectively. | Enhance NPs uptake | 212.6 ± 6.38 | 7.83 | 75.16 | (Verma et al., 2016) |
Additionally, transit time is another factor that affects the oral delivery and bioavailability of drugs to the colon. The normal transit time in the small intestine is approximately 4 h, with an inter-individual variability of 2 to 6 h; that of colon, however, is relatively variable, ranging from 6 to 70 h.
CANPs as a pH-sensitive ODDSs that protect insulin from damage and degradation in low pH environments, as well as gastrointestinal enzymes. Chen et al. used CANPs as carriers for the Cp1-11 peptide/insulin complex to protect insulin from damage and to enhance the biological activity of monomeric insulin (Chen et al., 2019). Their results indicate that encapsulation in CANPs is an effective way to protect the complex of insulin/Cp1-11 peptide. CANPs thus are one of the promising tactics to deliver insulin to treat type 1 diabetes patients. In another context, Alfatama et al. used alginate-C18 conjugated NPs loaded in TPP-linked chitosan oleic acid to improve mucus penetration and intracellular transport and to compensate for the shortcomings of alginate gels with high porosity and rapid drug release (Alfatama et al., 2018).
The core-shell structure is the most commonly used form of CANPs. Zhang et al. developed a core-shell CANPs based on positively charged chitosan and negatively charged alginate to functionally simulate the neutral surface of the virus formed through a highly negatively charged surface to increase the mucus permeability of the insulin-loaded nanocomplex (Zhang et al., 2018). The negatively charged alginate coating reduced the mucus penetration barrier of the nanocomposite and increased villi absorption and intestinal penetration. Bichitosan/albumin coated alginate-DSS-NPs further improved the mucosal adhesion efficiency and insulin permeability, so as to enhance the oral administration of insulin (Lopes et al., 2016). Approximately 70% of insulin was successfully retained in NPs in the gastric environment and then slowly released upon entry into the intestinal environments.
LBL coated CANPs can also be used for insulin protection. Zhang et al. developed an LBL-based oral insulin delivery system which is automatically formed by the ionic attraction between polygalacturonic acid (PGLA), chitosan, and alginate. The combination of particles avoided intestinal degradation caused by pH sensitivity and improved the overall blood sugar lowering effect (Zhang et al., 2018). Vitamin B12 was combined in LBL coated CANPs to improve the stability of insulin against gastric enzymes, and control insulin release to reach its physiological concentration (Verma et al., 2016). Li et al., prepared chitosan and TPP into NPs, and then further coated the surface of the nanoparticles with alginic acid and added calcium ions to form microbeads to protect insulin (Li et al., 2021). Such NPs showed excellent controlled-release properties in simulated stomach and small intestine solutions. The insulin-loaded CANPs were even capable of controlling hyperglycemia within 100 hours, which offers a potential therapeutic strategy for the treatment of diabetes.
Although some progress has been made over the years with oral insulin carriers, this work still requires further research before it can be truly used in the clinic. As mentioned above, the oral insulin delivery systems based on CANPs can achieve protection and transport of insulin and improve the physicochemical and biological stability and loading efficiency of insulin (Li et al., 2013). However, the utilization of oral insulin is still too low compared to intravenous subcutaneous injection. In addition, to maintain the stability of insulin in the gastrointestinal tract is also a crucial issue, especially for those oral delivery systems that use mucus adhesion systems to allow for extended insulin residence time (Wong, 2010). We believe that an ideal oral insulin delivery system should be biocompatible and biodegradable, and be able to remain stable in the gastrointestinal tract and achieve appropriate bioavailability.
Prospects and challenges
Application of colon-targeted oral drug delivery is unarguably the most convenient approach for the treatment of common intestinal diseases such as UC and CRC (Ling et al., 2019). The oral therapy of UC and CRC is a challenging task mainly due to its high complexity. There have been several research efforts in the field of colon-targeted drug delivery systems for the treatment of UC and CRC with varying degrees of success. Nonetheless, the effectiveness is significantly reduced due to enzymatic digestion in the stomach, make many challenges to efficiently deliver drugs to the colon (Yu et al., 2009). CANPs showed strong electrostatic interaction between alginate and chitosan, which leads to shrinkage and gel formation at low pH (in stomach), thereby protecting the drugs from GIT and the aggressive gastric environment (Ensign et al., 2012). Colon-targeted drug delivery of CANPs in UC and CRC have certain advantages, such as increased drug absorption, improved oral drug bioavailability and therapeutic efficiency, reduced systemic toxicity and reduced administered doses (Dos Santos et al., 2021).
The major obstacle for oral drug delivery via nanocarrier is the fact that it needs to cross several natural barriers of the human body before the incorporated drug can reach the target cell. Once ingested, CANPs will protect the drug from the acidic environment and proteolytic ‘thunderstorms’ in the stomach. After leaving the stomach, drug loaded CANPs enter the small intestine and are transported along the duodenum, jejunum, and ileum. The pH-dependence determines the drug loading and release rate of CANPs in colon. To improve the pH-dependence of CANPs material, Zhang et al. developed an automatic oral insulin delivery system formed by the ionic attraction between PGLA, chitosan and alginate which made CANPs more effective against gastric acid erosion in vitro experiments (Zhang et al., 2018). In addition, the report of enhancing the stability of CANPs in acidic environments by adding gelatin was also mentioned previously (Ceylan et al., 2021). Therefore, exploring more effective and nontoxic pH-stable materials is an important direction for CANPs as colon-specific therapies.
The current researches about colon-targeted delivery of CANPs have focused more on how to avoid the degradation of gastric acid, especially the pH-dependent release in vitro. However, after the drug enters the colon, it is still unclear whether it can play a normal pharmacological effect and be metabolized by the intestinal tract. 5-ASA, also known as mesalamine, is the treatment of choice for mild to moderate cases of UC. In our previous study, 5-ASA was encapsulated in microspheres prepared by CANPs-based hydrogels (Wu et al., 2021). This hydrogel microsphere had different swelling abilities in different pH media, which had colon-targeting and strong retention ability in colitis mice, and the microsphere was biodegradable, achieving a therapeutic effect superior to free drugs. However, whether this sustained-release system can overcome its extensive hepatic and intestinal metabolism, resulting in the formation of less active N-acetyl-5-ASA, has not been investigated. Thus, in addition to studying more effective oral drug delivery materials, more attention should be paid to the efficacy and absorption of loaded drugs in the colon in the future.
Moreover, some challenges remain in the clinical treatment of colon targeted drug delivery for intestinal disease. The location of this final GIT moiety presents a series of physical, chemical and enzymatic barriers that hinder the delivery and stability of oral drugs and is considered one of the major issues to overcome. At present, the application of CANPs is still at the verification of in vitro and animal experiments. CANPs still have a long way to go to overcome the several challenges facing oral therapeutic delivery and intestinal disease therapy.
Funding Statement
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the National Research Foundation of Korea (NRF-2016R1A5A1011974) and (NRF-2020R1A2C1102100).
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Alfatama M, Lim LY, Wong TW. (2018). Alginate-C18 conjugate nanoparticles loaded in tripolyphosphate-cross-linked chitosan-oleic acid conjugate-coated calcium alginate beads as oral insulin carrier. Mol Pharm 15:3369–82. [DOI] [PubMed] [Google Scholar]
- ALQuadeib BT, Eltahir EK, Alagili MF. (2020). The oral administration of lidocaine HCl biodegradable microspheres: formulation and optimization. Int J Nanomed 15:857–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aluani D, Tzankova V, Kondeva-Burdina M, et al. (2017). Еvaluation of biocompatibility and antioxidant efficiency of chitosan-alginate nanoparticles loaded with quercetin. Int J Biol Macromol 103:771–82. [DOI] [PubMed] [Google Scholar]
- Anderson DS, Patchin ES, Silva RM, et al. (2015). Influence of particle size on persistence and clearance of aerosolized silver nanoparticles in the rat lung. Toxicol Sci 144:366–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhunchu S, Rojsitthisak P. (2014). Biopolymeric alginate-chitosan nanoparticles as drug delivery carriers for cancer therapy. Pharmazie 69:563–70. [PubMed] [Google Scholar]
- Calvo P, Remuán-López C, Vila-Jato JL, Alonso MJ. (1997). Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. J Appl Polym Sci 63:125–32. [Google Scholar]
- Ceylan O, Karakus H, Cicek H. (2021). Design and in vitro antibiofilm activity of propolis diffusion-controlled biopolymers. Biotechnol Appl Biochem 68:789–800. [DOI] [PubMed] [Google Scholar]
- Chambin O, Dupuis G, Champion D, et al. (2006). Colon-specific drug delivery: Influence of solution reticulation properties upon pectin beads performance. Int J Pharm 321:86–93. [DOI] [PubMed] [Google Scholar]
- Chen MC, Sonaje K, Chen KJ, Sung HW. (2011). A review of the prospects for polymeric nanoparticle platforms in oral insulin delivery. Biomaterials 32:9826–38. [DOI] [PubMed] [Google Scholar]
- Chen T, Li S, Zhu W, et al. (2019). Self-assembly pH-sensitive chitosan/alginate coated polyelectrolyte complexes for oral delivery of insulin. J Microencapsul 36:96–107. [DOI] [PubMed] [Google Scholar]
- Chen X, Ren Y, Feng Y, et al. (2019). Cp1-11 peptide/insulin complex loaded pH-responsive nanoparticles with enhanced oral bioactivity. Int J Pharm 562:23–30. [DOI] [PubMed] [Google Scholar]
- Chen X, Zhao X, Wang G. (2020). Review on marine carbohydrate-based gold nanoparticles represented by alginate and chitosan for biomedical application. Carbohydr Polym 244:116311. [DOI] [PubMed] [Google Scholar]
- Chu JS, Wang ZJ. (2020). Protocol optimization for renal mass detection and characterization. Radiol Clin North Am 58:851–73. [DOI] [PubMed] [Google Scholar]
- Dos Santos AM, Carvalho SG, Meneguin AB, et al. (2021). Oral delivery of micro/nanoparticulate systems based on natural polysaccharides for intestinal diseases therapy: Challenges, advances and future perspectives. J Control Release 334:353–66. [DOI] [PubMed] [Google Scholar]
- Ensign LM, Cone R, Hanes J. (2012). Oral drug delivery with polymeric nanoparticles: the gastrointestinal mucus barriers. Adv Drug Deliv Rev 64:557–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glavas Dodov M, Calis S, Crcarevska MS, et al. (2009). Wheat germ agglutinin-conjugated chitosan-Ca-alginate microparticles for local colon delivery of 5-FU: development and in vitro characterization. Int J Pharm 381:166–75. [DOI] [PubMed] [Google Scholar]
- Glavas-Dodov M, Steffansen B, Crcarevska MS, Geskovski N, et al. (2013). Wheat germ agglutinin-functionalised crosslinked polyelectrolyte microparticles for local colon delivery of 5-FU: in vitro efficacy and in vivo gastrointestinal distribution. J Microencapsul 30:643–56. [DOI] [PubMed] [Google Scholar]
- Haddadzadegan S, Dorkoosh F, Bernkop-Schnurch A. (2022). Oral delivery of therapeutic peptides and proteins: technology landscape of lipid-based nanocarriers. Adv Drug Delivery Rev 182:114097. [DOI] [PubMed] [Google Scholar]
- Hagras NA, Mogahed N, Sheta E, et al. (2022). The powerful synergistic effect of spiramycin/propolis loaded chitosan/alginate nanoparticles on acute murine toxoplasmosis. PLoS Negl Trop Dis 16:e0010268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janardhanam LSL, Indukuri VV, Verma P, Dusane AC, et al. (2020). Functionalized layer-by-layer assembled film with directional 5-fluorouracil release to target colon cancer. Mater Sci Eng C Mater Biol Appl 115:111118. [DOI] [PubMed] [Google Scholar]
- Jindal AB. (2017). The effect of particle shape on cellular interaction and drug delivery applications of micro- and nanoparticles. Int J Pharm 532:450–65. [DOI] [PubMed] [Google Scholar]
- Jo DH, Kim JH, Lee TG, Kim JH. (2015). Size, surface charge, and shape determine therapeutic effects of nanoparticles on brain and retinal diseases. Nanomed Nanotechnol Biol Med 11:1603–11. [DOI] [PubMed] [Google Scholar]
- Kato Y, Ozawa S, Miyamoto C, et al. (2013). Acidic extracellular microenvironment and cancer. Cancer Cell Int 13:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppler K, Humpf HU. (2005). Metabolism of anthocyanins and their phenolic degradation products by the intestinal microflora. Bioorg Med Chem 13:5195–205. [DOI] [PubMed] [Google Scholar]
- Khorshidian N, Mahboubi A, Kalantari N, et al. (2019). Chitosan-coated alginate microcapsules loaded with herbal galactagogue extract: formulation optimization and characterization. Iran J Pharm Res 18:1180–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Siegmund B, Le Berre C, et al. (2020). Ulcerative colitis. Nat Rev Dis Primers 6:74. [DOI] [PubMed] [Google Scholar]
- Kumar P, Mishra B. (2008). Colon targeted drug delivery systems--an overview. Curr Drug Deliv 5:186–98. [DOI] [PubMed] [Google Scholar]
- Kyzioł A, Mazgała A, Michna J, et al. (2017). Preparation and characterization of alginate/chitosan formulations for ciprofloxacin-controlled delivery. J Biomater Appl 32:162–74. [DOI] [PubMed] [Google Scholar]
- Lang X, Wang T, Sun M, et al. (2020). Advances and applications of chitosan-based nanomaterials as oral delivery carriers: a review. Int J Biol Macromol 154:433–45. [DOI] [PubMed] [Google Scholar]
- Lanjhiyana SK, Bajpayee P, Kesavan K, et al. (2013). Chitosan-sodium alginate blended polyelectrolyte complexes as potential multiparticulate carrier system: colon-targeted delivery and gamma scintigraphic imaging. Expert Opin Drug Deliv 10:5–15. [DOI] [PubMed] [Google Scholar]
- Li J, Wu H, Jiang K, Liu Y, et al. (2021). Alginate calcium microbeads containing chitosan nanoparticles for controlled insulin release. Appl Biochem Biotechnol 193:463–78. [DOI] [PubMed] [Google Scholar]
- Li P, Nielsen HM, Fano M, Müllertz A. (2013). Preparation and characterization of insulin-surfactant complexes for loading into lipid-based drug delivery systems. J Pharm Sci 102:2689–98. [DOI] [PubMed] [Google Scholar]
- Li S, Jin M, Wu Y, et al. (2021). An efficient enzyme-triggered controlled release system for colon-targeted oral delivery to combat dextran sodium sulfate (DSS)-induced colitis in mice. Drug Deliv 28:1120–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Li W, Wan Y, et al. (2022). Preparation, characterization and releasing property of antibacterial nano-capsules composed of ε-PL-EGCG and sodium alginate-chitosan. Int J Biol Macromol 204:652–60. [DOI] [PubMed] [Google Scholar]
- Li YJ, Teng BH, Zhao YH, et al. (2019). [Preparation and evaluation of carboxymethyl chitosan/sodium alginate hydrogel for cartilage tissue engineering]. Hua Xi Kou Qiang Yi Xue Za Zhi 37:253–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling K, Wu H, Neish AS, Champion JA. (2019). Alginate/chitosan microparticles for gastric passage and intestinal release of therapeutic protein nanoparticles. J Control Release 295:174–86. [DOI] [PubMed] [Google Scholar]
- Lopes M, Shrestha N, Correia A, et al. (2016). Dual chitosan/albumin-coated alginate/dextran sulfate nanoparticles for enhanced oral delivery of insulin. J Control Release 232:29–41. [DOI] [PubMed] [Google Scholar]
- Manivasagan P, Bharathiraja S, Bui NQ, et al. (2016). Doxorubicin-loaded fucoidan capped gold nanoparticles for drug delivery and photoacoustic imaging. Int J Biol Macromol 91:578–88. [DOI] [PubMed] [Google Scholar]
- Mao Y, Zhao M, Ge Y, Fan J. (2016). Novel alginate-chitosan composite microspheres for implant delivery of vancomycin and in vivo evaluation. Chem Biol Drug Des 88:434–40. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay P, Chakraborty S, Bhattacharya S, et al. (2015). pH-sensitive chitosan/alginate core-shell nanoparticles for efficient and safe oral insulin delivery. Int J Biol Macromol 72:640–8. [DOI] [PubMed] [Google Scholar]
- Naeem M, Awan UA, Subhan F, et al. (2020). Advances in colon-targeted nano-drug delivery systems: challenges and solutions. Arch Pharm Res 43:153–69. [DOI] [PubMed] [Google Scholar]
- Naskar S, Koutsu K, Sharma S. (2019). Chitosan-based nanoparticles as drug delivery systems: a review on two decades of research. J Drug Targeting 27:379–93. [DOI] [PubMed] [Google Scholar]
- Nunthanid J, Huanbutta K, Luangtana-Anan M, et al. (2008). Development of time-, pH-, and enzyme-controlled colonic drug delivery using spray-dried chitosan acetate and hydroxypropyl methylcellulose. Eur J Pharm Biopharm 68:253–9. [DOI] [PubMed] [Google Scholar]
- Oshi MA, Lee J, Naeem M, et al. (2020). Curcumin nanocrystal/pH-responsive polyelectrolyte multilayer core-shell nanoparticles for inflammation-targeted alleviation of ulcerative colitis. Biomacromolecules 21:3571–81. [DOI] [PubMed] [Google Scholar]
- Renukuntla J, Vadlapudi AD, Patel A, et al. (2013). Approaches for enhancing oral bioavailability of peptides and proteins. Int J Pharm 447:75–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostami N, Nikkhoo A, Khazaei-Poul Y, et al. (2020). Coinhibition of S1PR1 and GP130 by siRNA-loaded alginate-conjugated trimethyl chitosan nanoparticles robustly blocks development of cancer cells. J Cell Physiol 235:9702–17. [DOI] [PubMed] [Google Scholar]
- Segal JP, LeBlanc JF, Hart AL. (2021). Ulcerative colitis: an update. Clin Med (Lond) 21:135–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segale L, Giovannelli L, Mannina P, Pattarino F. (2016). Calcium alginate and calcium alginate-chitosan beads containing celecoxib solubilized in a self-emulsifying phase. Scientifica 2016:5062706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinde UA, Shete JN, Nair HA, Singh KH. (2014). Design and characterization of chitosan-alginate microspheres for ocular delivery of azelastine. Pharm Dev Technol 19:813–23. [DOI] [PubMed] [Google Scholar]
- Sinha P, Udhumansha U, Rathnam G, et al. (2018). Capecitabine encapsulated chitosan succinate-sodium alginate macromolecular complex beads for colon cancer targeted delivery: in vitro evaluation. Int J Biol Macromol 117:840–50. [DOI] [PubMed] [Google Scholar]
- Suarato G, Li W, Meng Y. (2016). Role of pH-responsiveness in the design of chitosan-based cancer nanotherapeutics: a review. Biointerphases 11:04B201. 04b201. [DOI] [PubMed] [Google Scholar]
- Sudhakar S, Chandran SV, Selvamurugan N, Nazeer RA. (2020). Biodistribution and pharmacokinetics of thiolated chitosan nanoparticles for oral delivery of insulin in vivo. Int J Biol Macromol 150:281–8. [DOI] [PubMed] [Google Scholar]
- Taemeh MA, Shiravandi A, Korayem MA, Daemi H. (2020). Fabrication challenges and trends in biomedical applications of alginate electrospun nanofibers. Carbohydr Polym 228:115419. [DOI] [PubMed] [Google Scholar]
- Venkatesan J, Lee JY, Kang DS, et al. (2017). Antimicrobial and anticancer activities of porous chitosan-alginate biosynthesized silver nanoparticles. Int J Biol Macromol 98:515–25. [DOI] [PubMed] [Google Scholar]
- Verma A, Sharma S, Gupta PK, et al. (2016). Vitamin B12 functionalized layer by layer calcium phosphate nanoparticles: A mucoadhesive and pH responsive carrier for improved oral delivery of insulin. Acta Biomater 31:288–300. [DOI] [PubMed] [Google Scholar]
- Wen P, Hu TG, Li L, et al. (2018). A colon-specific delivery system for quercetin with enhanced cancer prevention based on co-axial electrospinning. Food Funct 9:5999–6009. [DOI] [PubMed] [Google Scholar]
- Wong TW. (2010). Design of oral insulin delivery systems. J Drug Target 18:79–92. [DOI] [PubMed] [Google Scholar]
- Wu Y, Li S, Jin M, et al. (2021). Preparation of MSZ hydrogel and its treatment of colitis. Front Pharmacol 12:706401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Su W, Xue Z, et al. (2021). Research on preparation of 5-ASA colon-specific hydrogel delivery system without crosslinking agent by mechanochemical method. Pharm Res 38:693–706. [DOI] [PubMed] [Google Scholar]
- Yoncheva K, Benbassat N, Zaharieva MM, et al. (2021). Improvement of the antimicrobial activity of oregano oil by encapsulation in chitosan-alginate nanoparticles. Molecules 26:7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You X, Kang Y, Hollett G, et al. (2016). Polymeric nanoparticles for colon cancer therapy: overview and perspectives. J Mater Chem B 4:7779–92. [DOI] [PubMed] [Google Scholar]
- Yu CY, Yin BC, Zhang W, et al. (2009). Composite microparticle drug delivery systems based on chitosan, alginate and pectin with improved pH-sensitive drug release property. Colloids Surf B Biointerfaces 68:245–9. [DOI] [PubMed] [Google Scholar]
- Yu S, Xu X, Feng J, et al. (2019). Chitosan and chitosan coating nanoparticles for the treatment of brain disease. Int J Pharm 560:282–93. [DOI] [PubMed] [Google Scholar]
- Yu X, Wen T, Cao P, et al. (2019). Alginate-chitosan coated layered double hydroxide nanocomposites for enhanced oral vaccine delivery. J Colloid Interface Sci 556:258–65. [DOI] [PubMed] [Google Scholar]
- Yue X, Yan B, Wang S, et al. (2020). Preparation of pH-responsive alginate-chitosan microspheres for L-valine loading and their effects on the A40926 production. Curr Microbiol 77:1016–23. [DOI] [PubMed] [Google Scholar]
- Zhang L, Qin H, Li J, et al. (2018). Preparation and characterization of layer-by-layer hypoglycemic nanoparticles with pH-sensitivity for oral insulin delivery. J Mater Chem B 6:7451–61. [DOI] [PubMed] [Google Scholar]
- Zhang X, Cheng H, Dong W, et al. (2018). Design and intestinal mucus penetration mechanism of core-shell nanocomplex. J Control Release 272:29–38. [DOI] [PubMed] [Google Scholar]
- Zhou X, Liu Y, Wang X, et al. (2020). Effect of particle size on the cellular uptake and anti-inflammatory activity of oral nanotherapeutics. Colloids Surf B Biointerfaces 187:110880. [DOI] [PubMed] [Google Scholar]