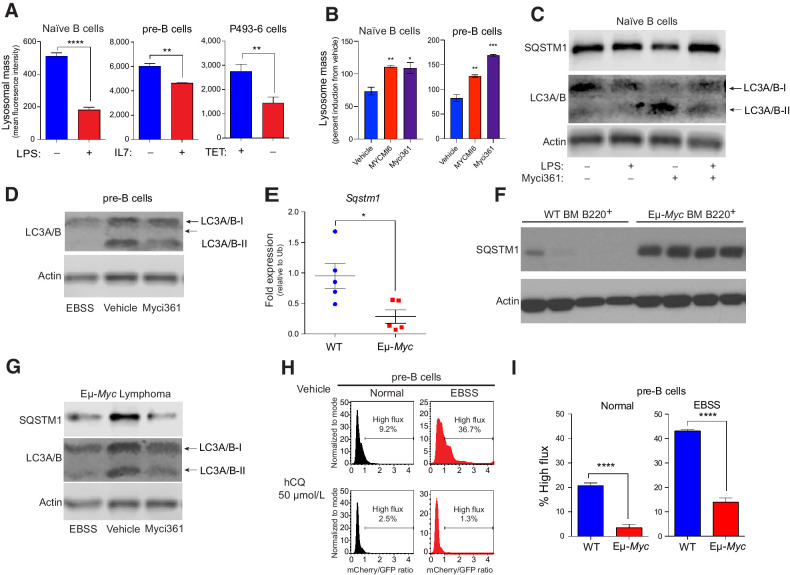
Figure 3.
MYC blocks autophagic flux. A, Mean fluorescent intensity of Lysotracker staining in (left to right): untreated versus LPS-treated (6 hours) naïve splenic mouse B cells; IL7-deprived (18 hours) pre-B cells that were then restimulated with IL7 for 6 hours; and P493-6 B lymphoma cells treated with TET for 48 hours and released from TET for 4 hours (n = 3 for all). B, Mean fluorescent intensity of LysoTracker staining of naïve B or pre-B cells treated with vehicle or with the Myc inhibitors Myci361 or MYCMI6 for 2 hours prior to stimulation with either LPS or IL7, respectively. C, Western blot analysis of SQSTM1 and LC3A/B isoforms in naïve splenic B220+ B cells that were left untreated or pretreated with Myci361 for 2 hours, followed by treatment with or without LPS for 4 hours. D, Western blot analysis of LC3A/B isoforms in pre-B cells (cultured in IL7 medium) that were treated with vehicle or with Myci361 for 2 hours, or that were shifted to EBSS medium for 6 hours. E and F, qRT-PCR (E) and Western blot (F) analysis of SQSTM1 levels in WT versus premalignant Eμ-Myc BM B220+ cells (n = 5 for E). G, Levels of SQSTM1 and LC3A/B isoforms levels in Eμ-Myc lymphoma after treatment with the Myc inhibitor Myci361 for 2 hours or following culture in EBSS media for 6 hours. Actin was used as a loading control for all immunoblots. H and I, Autophagic flux analyses in pre-B cells treated with 50 μmol/L hCQ (H), as measured in cells cultured in normal versus EBSS media for 6 hours, or in BM-derived WT versus Eμ-Myc pre-B cells (I). The ratio of GFP to mCherry from the retrovirally expressed mCherry-GFP-LC3 fusion protein was calculated and the percent of cells having high flux is shown (n = 3). Statistical analysis: A, B, E, and I, Student t tests were performed. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; ****, P ≤ 0.0001.