Figure 1. Themes in lectin–carbohydrate recognition.
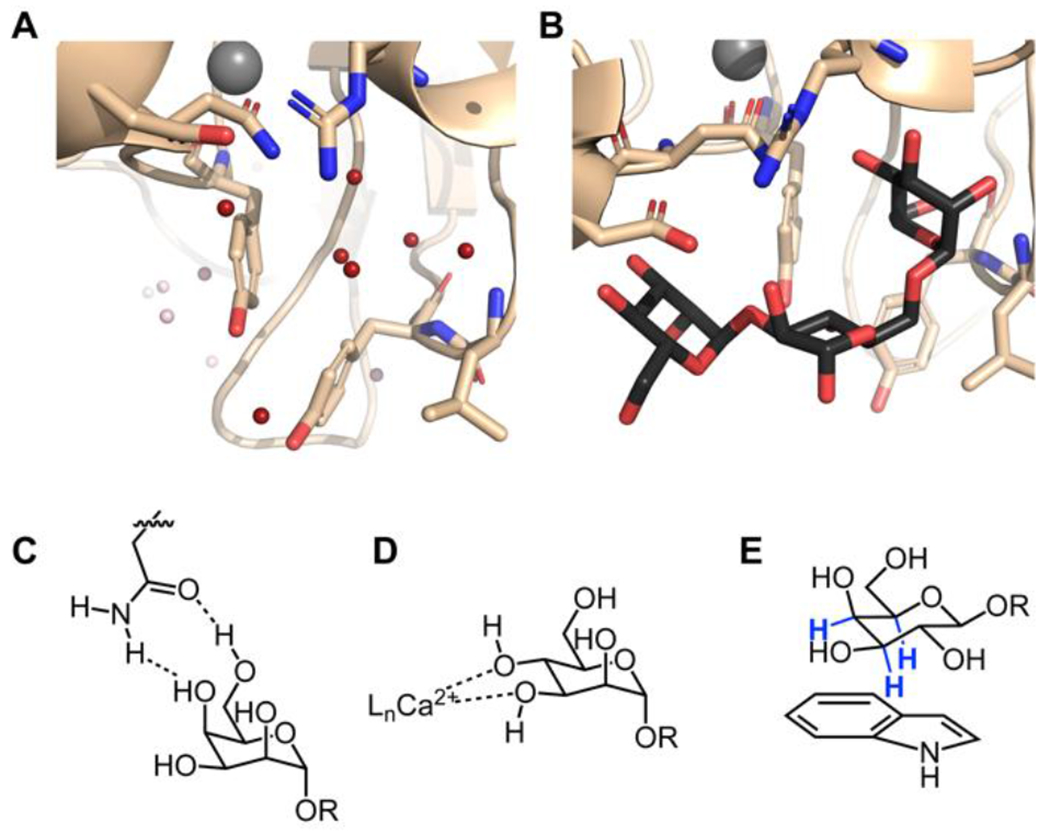
Structure of concanavalin A in the free (A) and bound (B) states. In view 1A, the water molecules displaced in the complex are highlighted in red (PDB code 1QNY).43 Concanavalin A bound to the core trimannoside is shown in B (PDB code 1CVN).34 C. Bifurcated hydrogen bonds orient the carbohydrate for binding. This binding can be entropically favorable because a single side-chain residue can displace two bound water molecules. D. Calcium ions coordinate hydroxyl groups and polarize the carbohydrate, enhancing the enthalpy of binding. This binding mode also displaces calcium ion-coordinated water molecules. C-type lectins and the intelectins use calcium coordination for ligand binding. E. CH-π interactions between the carbohydrate and aromatic residues in the protein are a crucial source of binding energy for highly polarized carbohydrates such as β-galactose. The C–H bonds highlighted in blue are those involved in the interaction.
