Figure 3. Linear free energy relationship analyzing chemical shift perturbation of β-galactoside protons in the presence of 5-substituted indoles.
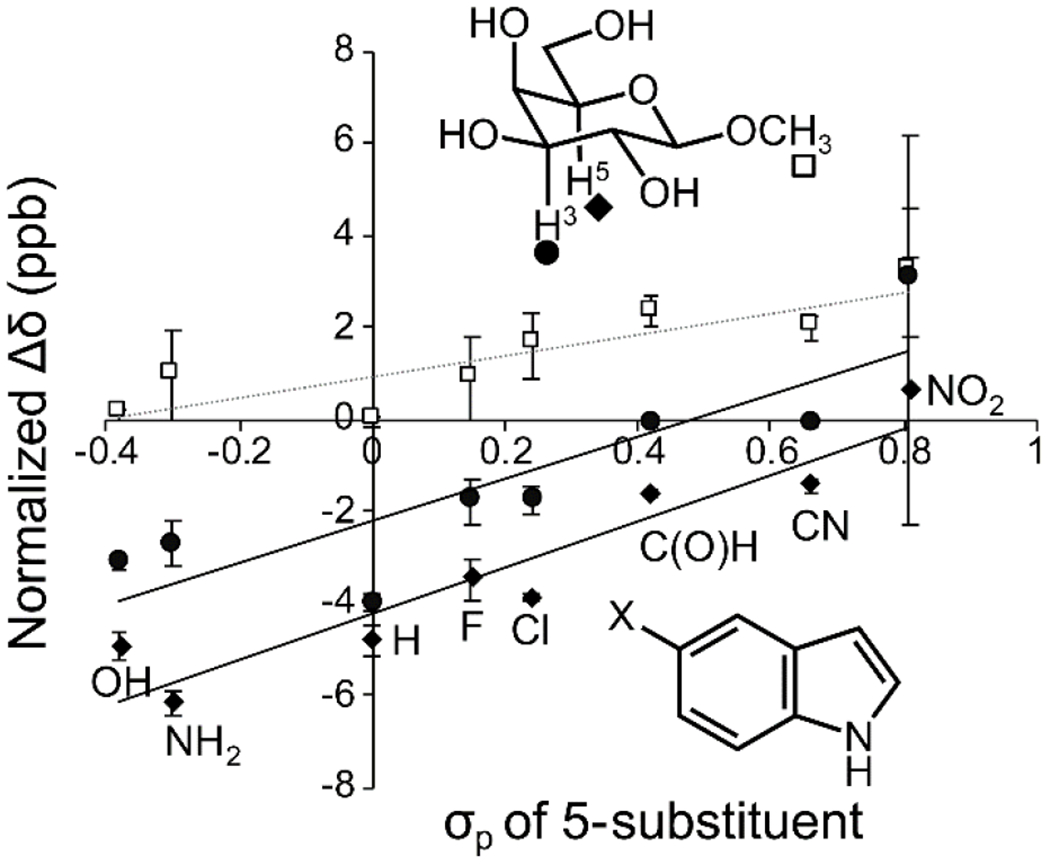
Indoles substituted with electron-donating groups have little effect on the methyl proteins (square) but induce higher upfield shifts of the interacting H3 (circle) and H5 (diamond) protons. The shifts are normalized to 10 mM indole using an observed linear relationship between concentration and chemical shift perturbation. Reproduced from Hudson, K. L.; Bartlett, G. J.; Diehl, R. C.; Agirre, J.; Gallagher, T.; Kiessling, L. L.; Woolfson, D. N., Carbohydrate-Aromatic Interactions in Proteins. J. Am. Chem. Soc. 2015, 137, 15152-15160. Copyright 2015 American Chemical Society.
