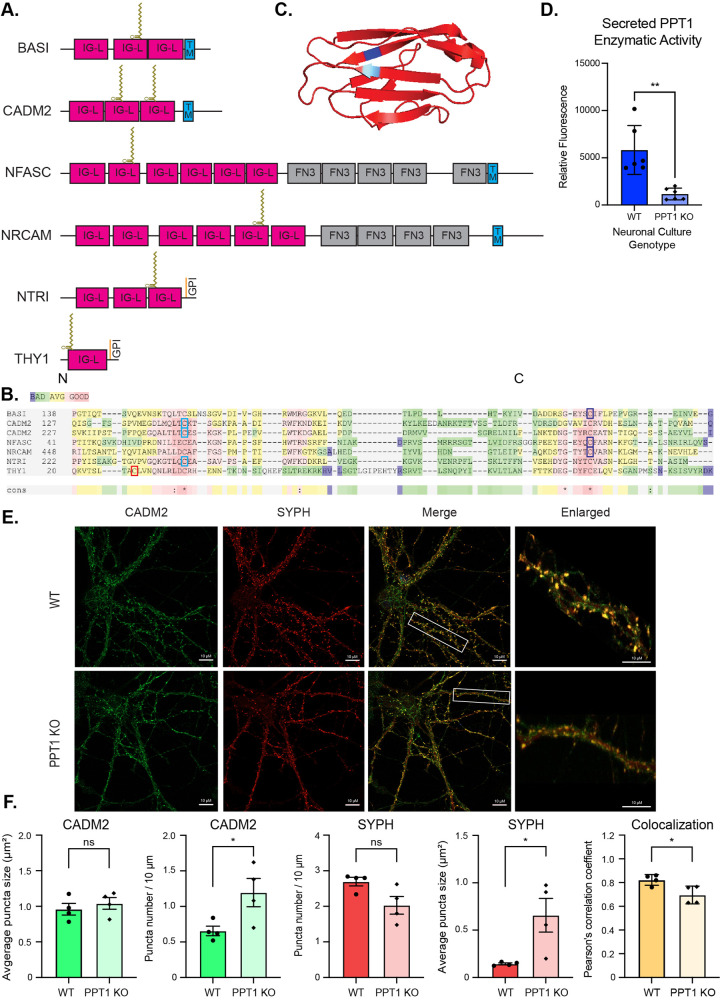
Fig 4. PPT1 depalmitoylates the IgG domain of synaptic adhesion molecules.
4. (A) Domain organization of IgG domain-containing synaptic adhesion molecules identified as high-confidence PPT1 substrates with palmitoylation sites represented as lipid chains. IG-L–Ig-like; FN3 –fibronectin 3; TM–transmembrane. (B) IgG domain-containing synaptic adhesion molecules show high homology surrounding the palmitoylated cysteines. Red indicates high homology; green indicates low homology. The 2 conserved cysteines at the N-terminal (light blue) and carboxyl terminus (dark blue) of this domain are both identified as carbamidomethylated in different experiments. (C) Representative IgG domain structure (CADM2; PDB, 3M45) with position of palmitoylated cysteines highlighted in blue. (D) Activity of PPT1 enzyme measured from WT or PPT1 KO primary neuronal culture medium. Data presented as mean relative fluorescence normalized to total protein concentration ± SD (n = 6 cultures per genotype; ** p > 0.01; S1 Data). (E) Endogenous syncam2 (CADM2) and synaptophysin 1 (SYPH) colocalize on MAP2+ neurites in WT and PPT1 KO mouse primary neuronal cultures (Scale bars: 10 μm; enlarged ROIs). (F) Quantifications of syncam2 and synaptophysin 1 puncta number (per 10-μm neurite segment), average puncta size (μm2), and colocalization (Pearson correlation coefficient). Data presented as mean of 5 ROIs per culture ± SD (n = 4 cultures; * p > 0.5; S1 Data). Ig, Immunoglobulin; KO, knockout; PPT1, palmitoyl protein thioesterase 1; ROS, region of interest; WT, wild-type.