Abstract
Background and Objective
The ovulatory menstrual cycle is characterized by hormonal fluctuations that influence physiological systems and functioning. Multi-sensor wearable devices can be sensitive tools capturing cycle-related physiological features pertinent to women’s health research. This study used the Oura ring to track changes in sleep and related physiological features, and also tracked self-reported daily functioning and symptoms across the regular, healthy menstrual cycle.
Methods
Twenty-six healthy women (age, mean (SD): 24.4 (1.1 years)) with regular, ovulatory cycles (length, mean (SD): 28.57 (3.8 days)) were monitored across a complete menstrual cycle. Four menstrual cycle phases, reflecting different hormone milieus, were selected for analysis: menses, ovulation, mid-luteal, and late-luteal. Objective measures of sleep, sleep distal skin temperature, heart rate (HR) and vagal-mediated heart rate variability (HRV, rMSSD), derived from the Oura ring, and subjective daily diary measures (eg sleep quality, readiness) were compared across phases.
Results
Wearable-based measures of sleep continuity and sleep stages did not vary across the menstrual cycle. Women reported no menstrual cycle-related changes in perceived sleep quality or readiness and only marginally poorer mood in the midluteal phase. However, they reported moderately more physical symptoms during menses (p < 0.001). Distal skin temperature and HR, measured during sleep, showed a biphasic pattern across the menstrual cycle, with increased HR (p < 0.03) and body temperature (p < 0.001) in the mid- and late-luteal phases relative to menses and ovulation. Correspondingly, rMSSD HRV tended to be lower in the luteal phase. Further, distal skin temperature was lower during ovulation relative to menses (p = 0.05).
Conclusion
The menstrual cycle was not accompanied by significant fluctuations in objective and perceived measures of sleep or in mood, in healthy women with regular, ovulatory menstrual cycles. However, other physiological changes in skin temperature and HR were evident and may be longitudinally tracked with the Oura ring in women over multiple cycles in a natural setting.
Keywords: sleep, wearables, menstrual cycle, ovulation, body temperature, women’s health
Plain Language Summary
This study aimed to use a wearable device (Oura ring), which has been previously tested against gold standard methods for sleep study, to examine changes in sleep, nocturnal heart rate and skin temperature, across four phases of the ovulatory menstrual cycle in 26 regularly menstruating, healthy women. We show that measures of sleep continuity and stages derived from the Oura ring stay relatively stable across menstrual cycle phases in women, consistent with their self-reported sleep assessments. On the other hand, distal skin temperature and heart rate, measured during sleep, increased in the luteal phase, consistent with ovulatory cycles. Skin temperature also showed a decrease in the ovulatory phase, likely reflecting the temperature-lowering effect of estrogen. Thus, wearable ring technology shows promise as a sensitive and informative tool for longitudinally tracking physiological changes across the menstrual cycle in women.
Introduction
A woman’s menstrual cycle is regulated by a complex interaction of reproductive hormones. Cycle-related hormonal changes can affect sleep, mood, and daily functioning.1,2 In reproductive-aged women, estrogen and progesterone levels fluctuate across a typical 28-day menstrual cycle, according to a predictable pattern.3 The first day of menstruation marks the start of the follicular phase, in which levels of estrogen and progesterone are low.3 During this phase, follicle-stimulating hormone (FSH) and luteinizing hormone (LH) levels start to increase, stimulating the development of several ovarian follicles, which produce estrogen (mainly estradiol).4 The increase in estrogen levels triggers a surge in LH, which stimulates ovulation around 12–16 hours later (around day 14; ovulation),5,6 although this time period can be longer for some women.7,8 Following ovulation, progesterone and estrogen levels are high (peaking 5–7 days after ovulation; mid-luteal phase), as the corpus luteum develops. In absence of implantation, the corpus luteum degenerates, and both progesterone and estrogen levels decline (late luteal phase) resulting in menstruation (the start of a new menstrual cycle).4
Beyond their reproductive function, these cycle-related hormonal changes influence other physiological systems, such as the autonomic nervous system, thermoregulation, and sleep. Resting HR increases and vagally-mediated heart rate variability decreases during the luteal phase in association with the rise in progesterone, compared with the follicular phase,9 an effect that is also evident during sleep.10 Resting state (basal) core body temperature also fluctuates in association with the hormone changes across the menstrual cycle, with a body temperature increase of about 0.4 °C in the luteal phase when progesterone is high (reviewed in)11. On the other hand, estrogen exerts a temperature-lowering effect such that a temperature drop around ovulation (coincident with the estrogen surge) may be detected. Increased body temperature correlates with increases in resting HR during the luteal phase.12 Critically, estrogen and progesterone receptors are present in central nervous system (CNS) areas involved in sleep regulation13,14 and have been shown to affect sleep regulation.15 Sleep disturbances are commonly reported by women in the late luteal and/or early follicular phase.16,17 However, when using more objective measures (eg, polysomnography [PSG]), this cycle-related variability in sleep continuity has not been observed,18 at least in young, healthy reproductive-aged women without menstrual-related complaints (see19 for a review).
When studying sleep in the context of the menstrual cycle, one of the main limitations of objective, in-lab studies is the feasibility for frequent sampling. Consequently, most studies only compare the early or mid-follicular phase (low-hormones) and the mid-luteal phase (high progesterone) of the cycle. Wearable sleep trackers offer an unprecedented opportunity to collect continuous data across a woman’s menstrual cycle in a natural, at-home setting. They also have an advantage over traditional research-grade actigraphy of including multiple sensors such as photoplethysmography (PPG), from which HR and HRV can be derived, and temperature sensors, in addition to an accelerometer for measuring activity.20 In fact, wearable devices have been recently used in research studies to monitor physiological changes, such as in temperature, across menstrual cycles, or as a fertility tool (ie, enabling prediction of ovulation windows).21–23 Considering female participants are sometimes excluded from clinical studies due to cycle-related variability,24 wearable technology could also be helpful to track and control for changes in physiological features across the menstrual cycle in the context of health research. The purpose of the present study was to use a wearable device (Oura ring), which has been previously tested against gold standard PSG,25,26,27 to examine changes in sleep and related physiological features, including nocturnal HR and temperature, across four different phases of the menstrual cycle in healthy reproductive-aged women. Changes in self-reported measures of sleep quality and daily functioning are also considered.
Method
Participants
This study is part of an ongoing study investigating the impact of sex hormones on sleep-dependent memory in reproductive-aged and late reproductive-aged women (RF1AG061355; Baker/Mednick). Only women between 18 and 35 years old who met the present study inclusion criteria (see Supplementary Table for details) constituted the final sample (N=26, see Table 1 for characteristics). The study complied with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008, and was approved by the University of California, Irvine, IRB committee (HS# 2018–4652). Participants were informed about the study and provided written informed consent. All participants were compensated $200 for completing the study.
Table 1.
Demographic Characteristics of the 26 Female Participants
| Age (Years), M, SD | 24.36 | 1.13 |
|---|---|---|
| Race/Ethnicity, N, % | ||
| White | 13 | 46.4 |
| Asian | 9 | 32.1 |
| Black/African American | 1 | 3.6 |
| Latino/Latina/Latinx | 1 | 3.6 |
| More than one race | 4 | 14.3 |
| Menstrual cycle length (days), M, SD | 28.57 | 3.8 |
| Menstrual cycle phase when Oura ring tracking started, N, % | ||
| Menses | 10 | 38.5 |
| Ovulation | 5 | 19.2 |
| Midluteal | 8 | 30.8 |
| Late luteal | 3 | 11.5 |
| Oura daily total sleep time across cycle (hours), M, SD | 7.04 | 1.25 |
Participants were recruited in California via social media adverts (eg, using age-sex targeted Facebook ads). First, all participants completed an online survey in which they were asked about demographic information, menstrual cycle history, sleep routines, and general health status. Research staff then contacted potentially eligible participants by video call to explain the study and complete the informed consent process. Sleep and health were further screened using an online battery of questionnaires hosted on Qualtrics. To this aim, we used the Insomnia Severity Index (ISI)28 for insomnia, the Berlin Questionnaire29 for sleep apnea, Generalized Anxiety Disorder 7-item (GAD-7)30 for anxiety, the Beck Depression Inventory (BDI)31 for clinical depression, and the Premenstrual Symptoms Screening Tool (PSST)32 for premenstrual symptoms. Only subjects who meet the inclusion criteria (Supplementary Table) were selected for the study. All participants had regular menstrual cycles (ie, menstrual cycle 22–35 days, menses <10 days) and were not using hormonal contraceptives. None of the participants had sleep disorders (eg, Insomnia, Obstructive Sleep Apnea) or any severe/chronic medical (eg, Diabetes, Hypertension), neurological (eg, Epilepsy, Traumatic Brain Injury) or psychiatric conditions (eg, Major Depressive disorder, Generalized Anxiety Disorder), and none of them was using medication known to affect sleep, the cardiovascular system, or brain function. Further, none of the women reported severe premenstrual symptoms or menstrual pain.
Procedure and Measures
Data presented here were collected between August 2020 and May 2021. The study consisted of a baseline phase and an experimental phase. The baseline phase lasted about a month, during which time participants completed a daily diary about their sleep and daily symptoms and marked days of menses and ovulation. At the end of the baseline phase, participants received the Oura ring and were instructed to wear it every day (24 hours) across the experimental phase. The experimental phase consisted of tracking physiological activity (Oura ring) and completing daily diaries across a complete menstrual cycle (eg, from the onset of menstruation of one cycle to the onset of menstruation of the next cycle). Participants started the experimental phase at different times across the menstrual cycle (see Table 1). Across the study, participants were encouraged to keep their regular sleep-wake routines.
Menstrual Cycle Tracking
Ovulation days were detected using a commercial urine test for luteinizing hormone (LH) (PREGMATE®, Ovulation Midstream Test Predictor Kit; sensitivity level: 25 mlU/mL). Participants were instructed to use ovulation tests, starting 5 days before the expected ovulation date and until 3 days after the first positive result. Results were visually checked by lab staff to confirm the positive LH surge.
Physiological Assessment Using the Oura Ring
Nocturnal sleep, HR, HRV, and skin temperature (distal body temperature) were continuously tracked using the Oura ring (Ōura Health, Oulu, Finland). The Oura ring is a commercial water-resistant multi-sensor wearable that measures HR, HRV, body temperature, respiration, and movement, via infrared photoplethysmography (PPG), negative temperature coefficient (NTC) thermistor, and a 3-D accelerometer, using proprietary algorithms. As reported by the manufacturer, the Oura ring calculates HRV as the square root of the mean squared differences of successive NN intervals (rMSSD), a known index of vagal activity,33 every 5 minutes throughout the night. The temperature sensor (NTC) is programmed to register skin temperature readings from the finger every minute, 24 hours a day, while the ring is worn. All sensors are located on the inside of the ring, being in contact with the finger. Oura team research showed that the Oura ring has a robust accuracy, sensitivity, and specificity (ranging from 74% to 98%) for wake-sleep detection and sleep staging.27 When tested against PSG in other research labs in healthy adolescents and young adults, the Oura ring showed good agreement with PSG in the whole night estimation of Total Sleep Time (TST) and Wake After Sleep Onset (WASO),25,26 with values comparable to research grade actigraphy. In other work, nocturnal skin temperature provided by Oura strongly correlated with oral temperature measured with a thermometer after wake-up.34
In this study, participants were provided with an Oura ring (firmware version 2.43.1) that fitted on the middle, ring, or index finger of their non-dominant hand. We ensured that each participant wore a correct Oura size ring that fit comfortably on her finger. Each participant was instructed via Zoom to create an anonymized Oura account linked to their subject identification number and to download the Oura App – a mobile phone application used to connect the ring to the participant’s phone via Bluetooth. Then, the participant gave consent to connect her Oura account to the Oura Cloud service. Participants were asked to synchronize the ring with the phone every morning by opening the Oura App. Data collected were accessible to the participant from the Oura App, and to lab staff through the Oura Cloud service. Data were checked daily to ensure data quality and successful acquisition; lab staff sent reminders or further instructions to the participant when needed. To ensure the ring battery level was optimal before bedtime, participants were asked to charge the ring daily during dinner time, setting up a charging routine. Participants were asked to not perform any firmware update or input any activity manually.
Self-Report Daily Measures
An online survey hosted on Qualtrics (Qualtrics International Inc.), a cloud-based software platform, was used to collect daily morning (Sleep Quality) and evening (Mood, Readiness, Physical symptoms) measures. Participants were instructed to complete the survey daily in the morning – just after waking up – and at night – right before going to sleep. The survey could be completed using any electronic device (eg, phone, tablet). Daily reminders with the survey link embedded were send to the participants by email. A description of the self-report daily measures is as follow: Sleep Quality – each morning, participants were asked to rate the sleep quality of the prior night, ranging from 0 (extremely bad) to 7 (extremely good); Mood – each evening, participants were asked to report how happy, worried/anxious, pleased, angry/hostile, frustrated, depressed/blue, joyful, unhappy, enjoyment/fun, and overwhelmed they felt throughout the day using a 7-point scale (Not at all/ Very slightly/Somewhat/Moderately/Much/Very much/Extremely). Positive feelings scored from 1 to 7, while negative feelings were reversed scored. A total score was obtained by summing all items, with higher scores indicating a more positive mood; Readiness – each evening, participants rated their daily overall alertness, mental stamina, physical endurance, physical strength, thinking, overall coordination, mood stability, and interactions with others, using a 5-point Likert scale (0 - Poor/ 1 - Fair/2 - Good/3 - Very good/4 - Excellent). An overall score was obtained by summing all items, with higher scores indicating more readiness; Physical symptoms – each evening, participants were asked to rate the level which they experienced pain, bloating, body ache, and tenderness during the day on a 5-point Likert scale (0 - Not at all/ 1 - A little/2 - A moderate amount/3 - A lot/4 - A great deal). A total score was obtained by summing all items, with higher scores indicating greater presence of physical symptoms during that day. Ratings were missing from one participant for these measures.
Data Management and Analysis
Menstrual Cycle Phases
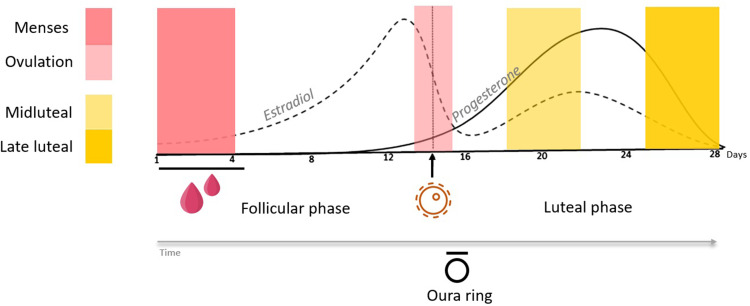
After data were collected, windows were calculated for each participant retrospectively, based on the menstruation and ovulation dates during the experimental phase, following the guidelines and method recently proposed by Schmalenberger et al.35 As outlined in Figure 1, four windows of interest were selected: two windows during the participant’s follicular phase: coded as Menses (4 days – starting at the onset of menses), and Ovulation (2 days – first day of a confirmed positive urine test plus the following day); and two windows during the luteal phase: coded as Midluteal (4 days – starting 6 days after the confirmed positive LH test) and Late luteal (4 days – starting four days before menstruation onset). Physiological, and self-report measures were averaged for each menstrual cycle phase.
Figure 1.
Representation of a typical 28-day ovulatory menstrual cycle, where estradiol and progesterone levels change over time. The first day of bleeding is considered Day 1. The Oura ring tracked the complete menstrual cycle in participants. Four menstrual phases (Menses, Ovulation, Midluteal and Late luteal) were selected as temporal windows of interest for analysis. Lines represent approximate hormone levels (hormone levels were not measured in the present study).
Sleep
First, lights-off (Oura “Bedtime start” – represents the time of bedtime onset) and lights-on (Oura “Bedtime end” – the time of sleep termination) were calculated. Then, standard sleep metrics were computed for each night based on the 30-s resolution data provided by the Oura ring:
Total Sleep Time (TST, min) – total amount of sleep (REM + light + deep) registered during the time in bed (period between light-off and lights-on) – was calculated as the sum of 30-seconds epochs classified as sleep x epoch length (s)/60.
Sleep Efficiency (SE, %) – percentage of total sleep time over time in bed – calculated as TST/time in bed.
Sleep Onset Latency (SOL, min) – detected latency from lights-off to the beginning of the first five minutes of persistent sleep – calculated as the sum of epochs classified as wake before the first epoch classified as sleep x epoch length (s)/60.
Wake After Sleep Onset (WASO, min) – period of wakefulness occurring after sleep onset – calculated as the sum of epochs classified as wake after the first sleep epoch × epoch length (s)/60.
Rapid-Eye-Movement (REM) Sleep (%) – percentage of REM sleep over total sleep time – calculated as (“REM”/60)/TST*100.
Deep Sleep (%) – percentage of deep sleep over total sleep time – calculated as (“deep”/60)/TST*100.
Light Sleep (%) – percentage of light sleep over total sleep time – calculated as (“light”/60)/TST*100.
Sleep Cardiac Autonomic Function
HR and HRV rMSSD were collected by the Oura ring during the sleep time (binned in 5-minutes consecutive intervals from the lights-off) and averaged to obtain mean HR (bpm) and HRV rMSSD (ms) for each night, independently from sleep stage transitions.
Sleep Skin Temperature
Although the Oura ring collects distal body temperature for a 24-hour period, we only used temperature measures sampled during sleep, as a more stable and reliable measure not affected by daily factors (eg, constant changes in the environment). The Sleep Skin Temperature was calculated as an average of the absolute distal body temperature (a measure directly provided by the Oura company) for a stable consolidated sleep period.
Statistical Analyses
Changes in objective physiological (Total Sleep Time, Sleep Efficiency, Sleep Onset Latency, Wake After Sleep Onset, REM sleep, Deep sleep, Light sleep, Sleep Skin Temperature, Sleep HR and HRV rMSSD from Oura ring) and self-reported data (Sleep Quality, Mood, Readiness, and Physical symptoms from daily diaries) across the four menstrual cycle phases (Menses, Ovulation, Midluteal and Late luteal) were statistically tested using Hierarchical linear models, accounting for between-woman random effects, using Stata (version SE 16.1 for Windows). Menses was the referent in each case. Estimated regression coefficients standard errors, and 95% confidence intervals (CI) for each phase indicator are provided in Table 2. For each model, additional post-hoc Wald tests were used to test for differences between ovulation, mid-luteal and late-luteal phases.
Table 2.
Results of Hierarchical Linear Regression Models Testing for Changes in Perceived Physical Symptoms, and Oura-Ring Derived Measures of Sleep Distal Temperature, Heart Rate (HR), and HRV, in 26 Young Women Across Four Phases of the Menstrual Cycle (Menses (Me), Ovulation (Ov), Midluteal (ML), and Late Luteal (LL). Menses is the Constant Term and Participant is Included as Random Effect
| Variable | Coefficient | SE | 95% CI |
|---|---|---|---|
| Perceived physical symptoms | |||
| Ovulation | −1.12* | 0.26 | [−1.65, −0.60] |
| Mid luteal | −1.07* | 0.26 | [−1.60, −0.55] |
| Late luteal | −0.68* | 0.26 | [−1.20, −0.16] |
| Distal skin temperature | |||
| Ovulation | −0.093* | 0.047 | [−.187, 0.001] |
| Mid luteal | 0.198* | 0.048 | [0.103, 0.293] |
| Late luteal | 0.242* | 0.047 | [0.148, 0.336] |
| Heart rate | |||
| Ovulation | 0.711 | 0.69 | [−.67, 2.09] |
| Mid luteal | 2.38* | 0.70 | [0.98, 3.78] |
| Late luteal | 2.49* | 0.69 | [1.11, 3.88] |
| rMSSD (vagal-related HRV) | |||
| Ovulation | −3.17 | 2.76 | [−8.66, 2.33] |
| Mid luteal | −5.47 | 2.79 | [−11.03, 0.097] |
| Late luteal | −5.96 | 2.76 | [−11.45, 0.47] |
Note: *Significantly different from menses (see text for details).
Abbreviations: CI, confidence interval; HRV, heart rate variability; rMSSD, square root of the mean squared differences of successive NN intervals; SE, standard error.
Results
Physiological Measures Tracked by the Oura Ring
Sleep
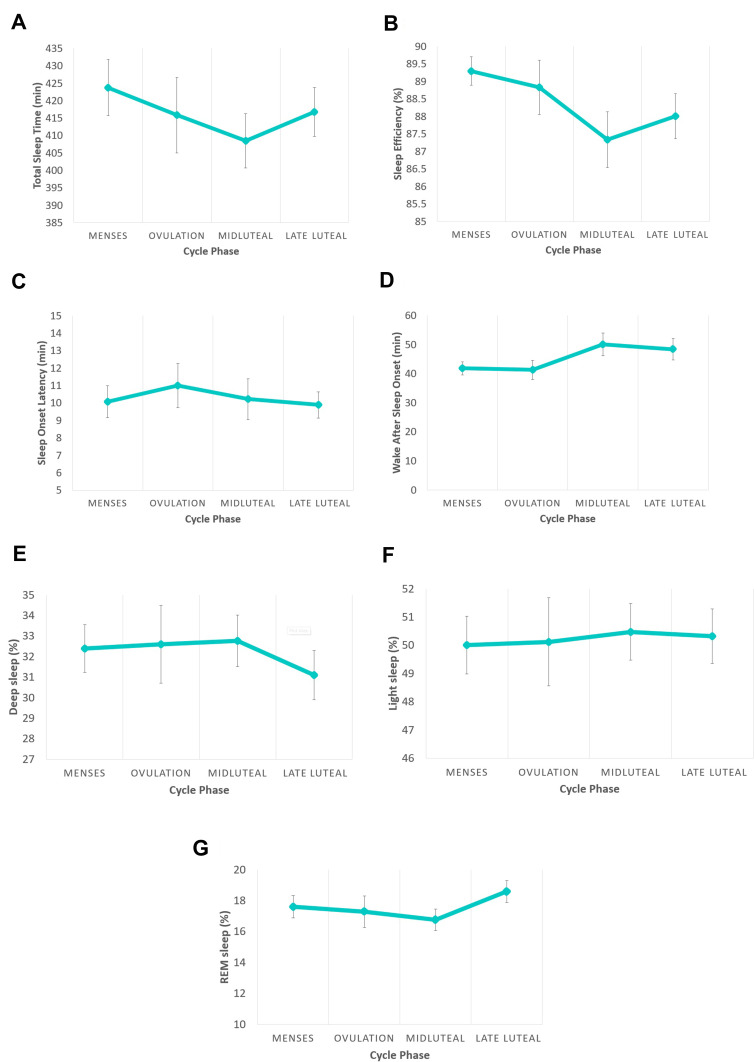
As shown in Figure 2, main sleep duration and quality features of Total Sleep Time (F (3, 74) = 0.92)), Sleep Onset Latency ((F (3, 74) = 0.18)), and Wake After Sleep Onset ((F (3, 74) = 2.15) did not significantly differ between the cycle phases. The overall model for Sleep Efficiency (F (3, 74) = 2.02, p=0.12) was also not significant. However, sleep efficiency was marginally lower (t = −2.28, p = 0.026) in the midluteal phase compared with menses (coefficient = −1.96, SE = 0.86, CI [−3.67, −0.246]). Sleep stages did not show any significant effect of menstrual cycle: REM Sleep (F (3, 74) = 1.97), Light Sleep (F (3, 74.0) = 0.46), Deep Sleep (F (3, 74) = 1.25).
Figure 2.
Main sleep features tracked with the Oura ring during four phases of the menstrual cycle (Menses, Ovulation, Midluteal and Late luteal) in healthy, reproductive-aged women (n = 26). Sleep measures included Total Sleep Time (A), Sleep efficiency (B), Sleep Onset Latency (C) Wake After Sleep Onset (D), Deep sleep (E), Light sleep (F), and Rapid-Eye-Movement (REM) sleep (G). The only menstrual cycle phase-related difference was in sleep efficiency (B), which was marginally lower in the midluteal phase compared with menses.
Sleep HR and Vagal-Related HRV (rMSSD)
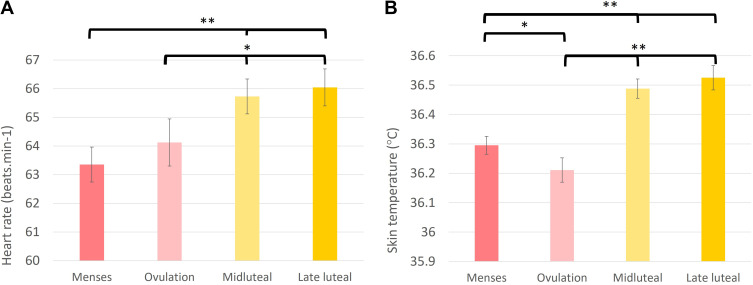
Nocturnal HR (F (3, 74) = 6.28, p<0.001) varied across the menstrual cycle (see Figure 3A and Table 2), showing an increase during the midluteal (t = 3.38, p=0.001) and late luteal (t = 3.59, p=0.001) phases compared to menses. Additional Wald tests showed that HR during ovulation was also lower than midluteal (X2 = 5.62, p=0.02) and late luteal (X2 = 6.59, p=0.01) phases, but did not differ between mid- and late-luteal phases. The overall model for rMSSD was not significant (F (3, 74) = 1.93, p=0.13). In the model, rMSSD was lower (t = −2.16, p=0.034) and tended to be lower (t = −1.96, p=0.054) in the late luteal and midluteal phases, respectively, compared with menses, although confidence intervals were wide (Table 2).
Figure 3.
Heart rate (A) and distal skin temperature (B) measured with the Oura ring during sleep showing significant differences between four phases of the menstrual cycle (Menses, Ovulation, Midluteal, and Late luteal) in healthy, reproductive-aged women (n = 26).
Notes: *p = 0.05; **p < 0.01.
Sleep Distal Skin Temperature
Sleep Skin Temperature was modulated across the menstrual cycle (F (3, 74) = 22.68, p<0.001; Figure 3B; Table 2). Specifically, temperature was higher in the midluteal (t = 4.14, p<0.001) and in the late luteal (t = 5.12, p<0.001) phases compared to menses. Also, as shown in Figure 3B, temperature was lower during ovulation (t = −1.98, p= 0.05) than during menses. Additional Wald tests showed that temperature during ovulation was also lower than midluteal (X2 = 37.2, p<0.001) and late luteal (X2 = 50.4, p<0.001) phases, but did not differ between mid- and late-luteal phases.
Daily Self-Reported Measures
Most of the self-reported measures did not significantly change across the menstrual cycle – Sleep Quality (F (3, 74) = 1.03) and Readiness (F (3, 70) = 0.47) did not vary. The overall model for Mood was not significant (F (3, 70) = 2.37, p = 0.08). However, Mood tended to be poorer in the midluteal phase compared with menses (t = −1.90, p = 0.061) and was poorer in the midluteal phase compared with ovulation (Wald test, X2 = 5.07, p = 0.02). Perceived Physical symptoms varied according to menstrual cycle phase (F (3, 70) = 7.81, p<0.001), being higher during menses compared with ovulation (t = −4.26, p<0.001), midluteal (t = −4.07, p<0.001), and late luteal (t = −2.62, p=0.01) phases (See Table 2 for details of statistical model).
Discussion
The purpose of the present study was to investigate changes in sleep and other physiological features across the natural menstrual cycle in reproductive-aged women in their home environment. To this end, we used a wearable device (the Oura ring), combined with self-reported daily measures of sleep, mood, and physical symptoms. There were no significant changes in self-reported sleep quality or in Oura indices of sleep duration, quality, and sleep stages in association with phases of the menstrual cycle (ie, menstruation, ovulation, mid- and late luteal), apart from a small decline in sleep efficiency in the midluteal phase, in healthy reproductive-aged women without menstrual-related complaints. These results concur with the majority of laboratory-based PSG studies in reproductive-aged women, including the landmark study by Driver et al,12,18 which investigated possible PSG and sleep EEG variation across multiple nights of the menstrual cycle. Using measures of core body temperature, urinary LH, and midluteal plasma levels of progesterone and estrogen to confirm menstrual cycle phases, these investigators found no significant variation across the menstrual cycle in TST, SE, SOL, WASO, or slow wave sleep (deep sleep). Similarly, perceived sleep quality and mood did not change across the menstrual cycle.18 There was, however, a dramatic increase in spindle frequency activity in the luteal phase,12,18 which has been replicated in other studies, and reflects increased sleep spindle density and duration compared with the follicular phase.36–38 These measures are only evident with detailed EEG analysis and are not measured with the Oura ring. Some PSG studies have also identified a small (~1%) reduction in REM sleep in the luteal phase (reviewed in19 however, this effect is small and was not detected with the Oura ring in our study.
There is an emerging body of literature on the use of technology to investigate the menstrual cycle.39–42 Some studies have used research-grade actigraphy, but to our knowledge, no studies published in peer-reviewed journals have used a wearable sleep tracker, to study sleep across the menstrual cycle. Only one research work, presented as conference proceedings, studied the relation between sleep and the menstrual cycle using a wrist-wearable device.43 Authors analyzed 541 cycles from 181 women and reported no change in total sleep across the phases of the menstrual cycle. However, contrary to our results, they found a significant decrease in deep sleep around menstruation. In an actigraphy study of 19 reproductive age women (18–43 years), subjective and objective sleep measures were not associated with estrogen or progesterone concentrations.44 Only a positive association between SE and estrogen metabolites (E1G), and a negative association between SE and progesterone metabolites (PdG) – yet both weak – were found. Another study45 of 163 late-reproductive-age women (~51 years) showed TST and SE as measured by actigraphy were significantly decreased in the premenstrual week, when compared to the previous week. We found only a small decline in SE in the midluteal phase, which may be magnified in midlife women. Indeed, we previously found that midlife women had more awakenings and arousals as assessed with PSG during a night in the luteal phase compared with the follicular phase.46 Differences in findings between studies could be attributed to differences in sample characteristics (eg age, presence/absence of menstrual-related problems), how menstrual cycle phases are operationalized, and use of different reference tests for ovulation in each study. A standard method to select the cycle phases is recommended when studying the menstrual cycle; some efforts have been done so far in this regard (e.g.35).
Considering the broader literature about changes in self-reported sleep across the menstrual cycle, several studies have noted a poorer sleep quality around menstruation (reviewed in)19. However, recent studies have highlighted the importance of considering menstrual cycle characteristics and symptom presence/severity as well as between-individual differences in patterns of sleep across the menstrual cycle. For example, Van Reen and Kiesner47 identified 3 patterns in the sleep-menstrual cycle phase relationship: while some women showed no cyclical change in perceived sleep difficulties, others showed an increase in sleep difficulties just before and during menstruation, and others showed a mid-cycle increase in perceived sleep difficulties. Further, menstrual cycle-related symptoms significantly contributed to difficulty sleeping.47 Sleep difficulties have also been reported to be more likely in women with irregular cycles,48 and in women with moderate to severe premenstrual mood symptoms.37,47,49 Overall, our findings indicate that, in the absence of substantial cycle-related changes in mood, subjective sleep quality and objective sleep duration and quality, as measured with the Oura ring, do not significantly vary between menstrual cycle phases in healthy, reproductive-aged women with regular cycles. Our results also show that self-reported physical symptoms, including pain, bloating, body aches, and tenderness are significantly more present during the menstruation phase in healthy women, however, these symptoms appeared not to affect sleep, likely because they were perceived in a moderate range. In contrast, the presence of more severe menstrual pain is associated with poorer sleep quality50 and reduced daily quality of life.51
In contrast to our finding of minimal changes in sleep, we found menstrual-cycle related variation in other physiological measures derived from the Oura ring. Distal skin temperature and heart rate, measured during sleep, were both increased, along with a trend for lower vagal-mediated HRV, in the luteal phase, which supports well-established findings in the literature based on measurements with research-grade devices.11,12,52 The reverse bi-phasic pattern between HR and HRV across the menstrual cycle seen in our data (ie, increased HR and trend for decreased vagal-mediated HRV, in the luteal phases compared to menses) has also been captured by wrist-worn devices.22,53 Our data, therefore, support the use of a wearable ring device to monitor ovulatory cycle-related physiological changes. Of particular relevance to fertility tracking, we found that the Oura ring also captured a significant distal skin temperature drop around ovulation. While less well studied than the luteal phase increase in body temperature, laboratory studies have found a drop in core body temperature in association with high estrogen.54,55 In a study in which the nocturnal wrist skin temperature was examined with a wearable,21 the cycle-related biphasic pattern was captured in 82% of the cycles, however, the temperature nadir associated with ovulation was not accurately detected (only 41% of cycles). Possibly, finger skin temperature may be more sensitive to smaller temperature changes such as occurs around ovulation. Other analyses of data from the Oura ring have also supported its potential use for fertility assessment: Using wavelet analyses, investigators found a consistent pattern of daytime distal skin temperature and HRV ultradian rhythms (2–5 h) power that anticipated the LH surge in all individuals (45 premenopausal and 10 perimenopausal women).23
One of the strengths of the present at-home-based study is the ecological validity of the objective sleep and sleep-related physiological measures made across a woman’s menstrual cycle. In contrast to in-lab PSG studies, in which participants must sleep overnight in an unusual sleep environment while being wired to be monitored, the use of a multi-sensory wearable allowed us to track objective sleep in a more natural and less invasive way. In-lab studies offer a reliable, robust, and more complete source of information about the physiological micro- and macro-structure of sleep, but wearables bring unprecedented advantages when studying sleep in the context of the menstrual cycle.40–42 While in-lab visits need to be scheduled prospectively across the cycle, and frequency of sampling is limited due to logistical constraints, in our study four customized windows for each participant could be retrospectively selected based on the participant’s specific menstrual cycle phases, owing to the continuous measures provided by the Oura ring. In this way, we also controlled the between-subject menstrual cycle variability (eg, different cycle length), an important factor that limits some studies when matching physiological changes (eg, body temperature) to menstrual cycle-related hormone changes.12 Lastly, anovulatory cycles happen about 28% of the time in women aged 20 to 24 years.56 By including in the study only participants who got a positive (confirmed) urine test for LH, we assured all the cycles examined were ovulatory.
The present study also has limitations. As previously shown, different factors may affect the accuracy of a multi-sensor wearable device, including device position, demographic factors (eg, age), alcohol consumption, amount of sleep disruption (for review, see20,57). Also, different levels of accuracy exist for different sleep outputs provided by these devices (eg, greater accuracy for sleep vs wake assessment, lower accuracy in “deep sleep” estimation) since outputs are derived from the various combinations of peripheral sensors, and challenges exist for the automatic determination of bedtime and wake time intervals.20,57 Thus, we cannot completely exclude the possibility that the Oura ring may exhibit different levels of accuracy across different menstrual cycle phases and sleep outcomes, potentially masking true menstrual-cycle effects on sleep. Additional work is needed to compare the accuracy of Oura ring with gold-standard PSG measures in women at different menstrual cycle phases to address some of these questions. A further limitation of our study was that blood hormonal levels were not collected, which would have been a more precise method to assign menstrual cycle phases. This limitation also makes it impossible to establish specific relationships between sleep and other physiological measures with hormonal levels across the cycle. One menstrual cycle per participant was used for the present study; thus, possible intra-subject variability between menstrual cycles needs to be considered in future work. Another limitation to consider is the relatively small sample size and homogeneity of the sample in terms of race and ethnicity, since most of our participants were white women. This is a frequent limitation in wearable devices research, which tends to overrepresent white race because photoplethysmographic (PPG) light signaling is not as accurate in people with darker skin tones.58 Sleep difficulties seem to be reported more frequently in racial/ethnic minorities,59 but possible differences in sleep and its physiological features across the menstrual cycle among race/ethnicity groups cannot be established in the present study. Sleep difficulties have also been reported to be more common in women approaching menopause.60,61 Future studies, in which wearable technology can play an important role, should investigate daily objective sleep and related physiological and self-reported measures across the menstrual cycle in perimenopausal women.
In conclusion, results from this study indicate that objective wearable-based measures of sleep continuity show minimal variability across the natural, ovulatory menstrual cycle in reproductive-aged women. On the other hand, there were phase-based shifts in nocturnal skin temperature and HR, including a drop in skin temperature around ovulation. Thus, wearable technology seems to be a sensitive and informative tool for tracking physiological changes that reflect ovulatory menstrual cycles. In comparison to in-lab, or self-report measures that are subject to biases, wearable technology holds promise as an objective tool to continuously study sleep and other physiological measures across the menstrual cycle in women in natural environments.
Acknowledgments
We thank all the participants for their time and research staff who helped collect the data.
Funding Statement
This study was supported by the National Institutes of Health (NIH) grant RF1AG061355 (Baker/Mednick). The content is solely the responsibility of the authors and does not necessarily represent the official views the National Institutes of Health.
Disclosure
Authors declare no conflict of interest related to the current work. Dr Massimiliano de Zambotti and Dr Fiona Baker have received research funding unrelated to this work from Noctrix Health, Inc., Verily Life Sciences LLC., and Lisa Health Inc. Dr de Zambotti is a co-founder of Lisa Health Inc. Dr de Zambotti and Dr Baker have ownership of shares in Lisa Health.
References
- 1.Laessle RG, Tuschl RJ, Schweiger U, Pirke KM. Mood changes and physical complaints during the normal menstrual cycle in healthy young women. Psychoneuroendocrinology. 1990;15(2):131–138. doi: 10.1016/0306-4530(90)90021-Z [DOI] [PubMed] [Google Scholar]
- 2.de Zambotti M, Colrain IM, Baker FC. Interaction between reproductive hormones and physiological sleep in women. J Clin Endocrinol Metab. 2015;100(4):1426–1433. doi: 10.1210/jc.2014-3892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hawkins SM, Matzuk MM. The menstrual cycle: basic biology. Ann N Y Acad Sci. 2008;1135(1):10–18. doi: 10.1196/annals.1429.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Messinis IE, Messini CI, Dafopoulos K. Novel aspects of the endocrinology of the menstrual cycle. Reprod Biomed Online. 2014;28(6):714–722. doi: 10.1016/j.rbmo.2014.02.003 [DOI] [PubMed] [Google Scholar]
- 5.Faust L, Bradley D, Landau E, et al. Findings from a mobile application-based cohort are consistent with established knowledge of the menstrual cycle, fertile window, and conception. Fertil Steril. 2019;112(3):450–457.e453. doi: 10.1016/j.fertnstert.2019.05.008 [DOI] [PubMed] [Google Scholar]
- 6.Su HW, Yi YC, Wei TY, Chang TC, Cheng CM. Detection of ovulation, a review of currently available methods. Bioeng Transl Med. 2017;2(3):238–246. doi: 10.1002/btm2.10058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller PB, Soules MR. The usefulness of a urinary LH kit for ovulation prediction during menstrual cycles of normal women. Obstet Gynecol. 1996;87(1):13–17. doi: 10.1016/0029-7844(95)00352-5 [DOI] [PubMed] [Google Scholar]
- 8.Stanford JB, White GL, Hatasaka H. Timing intercourse to achieve pregnancy: current evidence. Obstet Gynecol. 2002;100(6):1333–1341. doi: 10.1016/s0029-7844(02)02382-7 [DOI] [PubMed] [Google Scholar]
- 9.Schmalenberger KM, Eisenlohr-Moul TA, Jarczok MN, et al. Menstrual cycle changes in vagally-mediated heart rate variability are associated with progesterone: evidence from two within-person studies. J Clin Med. 2020;9(3):617. doi: 10.3390/jcm9030617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Zambotti M, Nicholas CL, Colrain IM, Trinder JA, Baker FC. Autonomic regulation across phases of the menstrual cycle and sleep stages in women with premenstrual syndrome and healthy controls. Psychoneuroendocrinology. 2013;38(11):2618–2627. doi: 10.1016/j.psyneuen.2013.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baker FC, Siboza F, Fuller A. Temperature regulation in women: effects of the menstrual cycle. Temperature. 2020;7(3):226–262. doi: 10.1080/23328940.2020.1735927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Driver HS, Werth E, Dijk DJ, Borbély AA. The menstrual cycle effects on sleep. Sleep Med Clin. 2008;3(1):1–11. doi: 10.1016/j.jsmc.2007.10.003 [DOI] [Google Scholar]
- 13.Curran-Rauhut MA, Petersen SL. The distribution of progestin receptor mRNA in rat brainstem. Brain Res Gene Expr Patterns. 2002;1(3–4):151–157. doi: 10.1016/S1567-133X(02)00011-X [DOI] [PubMed] [Google Scholar]
- 14.Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388(4):507–525. doi: [DOI] [PubMed] [Google Scholar]
- 15.Mong JA, Cusmano DM. Sex differences in sleep: impact of biological sex and sex steroids. Philos Trans R Soc Lond B Biol Sci. 2016;371(1688):20150110. doi: 10.1098/rstb.2015.0110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baker FC, Driver HS. Self-reported sleep across the menstrual cycle in young, healthy women. J Psychosom Res. 2004;56(2):239–243. doi: 10.1016/S0022-3999(03)00067-9 [DOI] [PubMed] [Google Scholar]
- 17.Kravitz HM, Janssen I, Santoro N, et al. Relationship of day-to-day reproductive hormone levels to sleep in midlife women. Arch Intern Med. 2005;165(20):2370–2376. doi: 10.1001/archinte.165.20.2370 [DOI] [PubMed] [Google Scholar]
- 18.Driver HS, Dijk DJ, Werth E, Biedermann K, Borbely AA. Sleep and the sleep electroencephalogram across the menstrual cycle in young healthy women. J Clin Endocrinol Metab. 1996;81(2):728–735. doi: 10.1210/jcem.81.2.8636295 [DOI] [PubMed] [Google Scholar]
- 19.Baker FC, Lee KA. Menstrual cycle effects on sleep. Sleep Med Clin. 2018;13(3):283–294. doi: 10.1016/j.jsmc.2018.04.002 [DOI] [PubMed] [Google Scholar]
- 20.de Zambotti M, Cellini N, Menghini L, Sarlo M, Baker FC. Sensors capabilities, performance, and use of consumer sleep technology. Sleep Med Clin. 2020;15(1):1–30. doi: 10.1016/j.jsmc.2019.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shilaih M, Goodale BM, Falco L, Kübler F, De clerck V, Leeners B. Modern fertility awareness methods: wrist wearables capture the changes in temperature associated with the menstrual cycle. Biosci Rep. 2018;38(6). doi: 10.1042/BSR20171279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goodale BM, Shilaih M, Falco L, Dammeier F, Hamvas G, Leeners B. Wearable sensors reveal menses-driven changes in physiology and enable prediction of the fertile window: observational study. J Med Internet Res. 2019;21(4):e13404. doi: 10.2196/13404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grant AD, Newman M, Kriegsfeld LJ. Ultradian rhythms in heart rate variability and distal body temperature anticipate onset of the luteinizing hormone surge. Sci Rep. 2020;10(1):20378. doi: 10.1038/s41598-020-76236-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor HA. Inclusion of women, minorities, and children in clinical trials: opinions of research ethics board administrators. J Empir Res Hum Res Ethics. 2009;4(2):65–73. doi: 10.1525/jer.2009.4.2.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chee N, Ghorbani S, Golkashani HA, Leong RLF, Ong JL, Chee MWL. Multi-night validation of a sleep tracking ring in adolescents compared with a research Actigraph and Polysomnography. Nat Sci Sleep. 2021;13:177–190. doi: 10.2147/NSS.S286070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Zambotti M, Rosas L, Colrain IM, Baker FC. The sleep of the ring: comparison of the OURA sleep tracker against polysomnography. Behav Sleep Med. 2019;17(2):124–136. doi: 10.1080/15402002.2017.1300587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Altini M, Kinnunen H. The promise of sleep: a multi-sensor approach for accurate sleep stage detection using the oura ring. Sensors. 2021;21(13):4302. doi: 10.3390/s21134302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. doi: 10.1016/S1389-9457(00)00065-4 [DOI] [PubMed] [Google Scholar]
- 29.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131(7):485–491. doi: 10.7326/0003-4819-131-7-199910050-00002 [DOI] [PubMed] [Google Scholar]
- 30.Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–1097. doi: 10.1001/archinte.166.10.1092 [DOI] [PubMed] [Google Scholar]
- 31.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4(6):561–571. doi: 10.1001/archpsyc.1961.01710120031004 [DOI] [PubMed] [Google Scholar]
- 32.Steiner M, Macdougall M, Brown E. The premenstrual symptoms screening tool (PSST) for clinicians. Arch Women’s Ment Health. 2003;6(3):203–209. doi: 10.1007/s00737-003-0018-4 [DOI] [PubMed] [Google Scholar]
- 33.de Zambotti M, Trinder J, Silvani A, Colrain IM, Baker FC. Dynamic coupling between the central and autonomic nervous systems during sleep: a review. Neurosci Biobehav Rev. 2018;90:84–103. doi: 10.1016/j.neubiorev.2018.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maijala A, Kinnunen H, Koskimaki H, Jamsa T, Kangas M. Nocturnal finger skin temperature in menstrual cycle tracking: ambulatory pilot study using a wearable Oura ring. BMC Women's Health. 2019;19(1):150. doi: 10.1186/s12905-019-0844-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmalenberger KM, Tauseef HA, Barone JC, et al. How to study the menstrual cycle: practical tools and recommendations. Psychoneuroendocrinology. 2021;123:104895. doi: 10.1016/j.psyneuen.2020.104895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baker FC, Kahan TL, Trinder J, Colrain IM. Sleep quality and the sleep electroencephalogram in women with severe premenstrual syndrome. Sleep. 2007;30(10):1283–1291. doi: 10.1093/sleep/30.10.1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baker FC, Sassoon SA, Kahan T, et al. Perceived poor sleep quality in the absence of polysomnographic sleep disturbance in women with severe premenstrual syndrome. J Sleep Res. 2012;21(5):535–545. doi: 10.1111/j.1365-2869.2012.01007.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ishizuka Y, Pollak CP, Shirakawa S, et al. Sleep spindle frequency changes during the menstrual cycle. J Sleep Res. 1994;3(1):26–29. doi: 10.1111/j.1365-2869.1994.tb00100.x [DOI] [PubMed] [Google Scholar]
- 39.Symul L, Holmes S. Labeling self-tracked menstrual health records with hidden semi-Markov models. Medrxiv. 2021. doi: 10.1101/2021.01.11.21249605 [DOI] [PubMed] [Google Scholar]
- 40.Symul L, Wac K, Hillard P, Salathé M. Assessment of menstrual health status and evolution through mobile apps for fertility awareness. NPJ Digit Med. 2019;2(1):64. doi: 10.1038/s41746-019-0139-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li K, Urteaga I, Shea A, Vitzthum VJ, Wiggins CH, Elhadad N. A predictive model for next cycle start date that accounts for adherence in menstrual self-tracking. J Am Med Inform Assoc. 2021;29(1):3–11. doi: 10.1093/jamia/ocab182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li K, Urteaga I, Wiggins CH, et al. Characterizing physiological and symptomatic variation in menstrual cycles using self-tracked mobile-health data. NPJ Digit Med. 2020;3(1):79. doi: 10.1038/s41746-020-0269-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shilaih M, Falco L, Kuebler F, Hamvas G, Cronin M, Leeners B. Monitoring sleep patterns change across the menstrual cycle using wearable sensors. Intern J Obstet Gynecol., 143, S3 (Abstracts of the XXII FIGO World Congress of Gynecology & Obstetrics, October 2018), Pg: 348. [Google Scholar]
- 44.Li DX, Romans S, De Souza MJ, Murray B, Einstein G. Actigraphic and self-reported sleep quality in women: associations with ovarian hormones and mood. Sleep Med. 2015;16(10):1217–1224. doi: 10.1016/j.sleep.2015.06.009 [DOI] [PubMed] [Google Scholar]
- 45.Zheng H, Harlow SD, Kravitz HM, et al. Actigraphy-defined measures of sleep and movement across the menstrual cycle in midlife menstruating women: study of Women’s Health Across the Nation Sleep Study. Menopause. 2015;22(1):66–74. doi: 10.1097/GME.0000000000000249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Zambotti M, Willoughby AR, Sassoon SA, Colrain IM, Baker FC. Menstrual cycle-related variation in physiological sleep in women in the early menopausal transition. J Clin Endocrinol Metab. 2015;100(8):2918–2926. doi: 10.1210/jc.2015-1844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Reen E, Kiesner J. Individual differences in self-reported difficulty sleeping across the menstrual cycle. Arch Women's Ment Health. 2016;19(4):599–608. doi: 10.1007/s00737-016-0621-9 [DOI] [PubMed] [Google Scholar]
- 48.Hachul H, Andersen ML, Bittencourt LR, Santos-Silva R, Conway SG, Tufik S. Does the reproductive cycle influence sleep patterns in women with sleep complaints? Climacteric. 2010;13(6):594–603. doi: 10.3109/13697130903450147 [DOI] [PubMed] [Google Scholar]
- 49.Gupta R, Lahan V, Bansal S. Subjective sleep problems in young women suffering from premenstrual dysphoric disorder. N Am J Med Sci. 2012;4(11):593–595. doi: 10.4103/1947-2714.103326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baker FC, Driver HS, Rogers GG, Paiker J, Mitchell D. High nocturnal body temperatures and disturbed sleep in women with primary dysmenorrhea. Am J Physiol. 1999;277(6):E1013–1021. doi: 10.1152/ajpendo.1999.277.6.E1013 [DOI] [PubMed] [Google Scholar]
- 51.Iacovides S, Avidon I, Bentley A, Baker FC. Reduced quality of life when experiencing menstrual pain in women with primary dysmenorrhea. Acta Obstet Gynecol Scand. 2014;93(2):213–217. doi: 10.1111/aogs.12287 [DOI] [PubMed] [Google Scholar]
- 52.Schmalenberger KM, Eisenlohr-Moul TA, Wurth L, et al. A systematic review and meta-analysis of within-person changes in cardiac vagal activity across the menstrual cycle: implications for female health and future studies. J Clin Med. 2019;8(11):1946. doi: 10.3390/jcm8111946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shilaih M, Clerck V, Falco L, Kübler F, Leeners B. Pulse rate measurement during sleep using wearable sensors, and its correlation with the menstrual cycle phases, a prospective observational study. Sci Rep. 2017;7(1):1294. doi: 10.1038/s41598-017-01433-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cagnacci A, Volpe A, Paoletti AM, Melis GB. Regulation of the 24-hour rhythm of body temperature in menstrual cycles with spontaneous and gonadotropin-induced ovulation. Fertil Steril. 1997;68(3):421–425. doi: 10.1016/S0015-0282(97)00242-2 [DOI] [PubMed] [Google Scholar]
- 55.Stephenson LA, Kolka MA. Esophageal temperature threshold for sweating decreases before ovulation in premenopausal women. J Appl Physiol. 1999;86(1):22–28. doi: 10.1152/jappl.1999.86.1.22 [DOI] [PubMed] [Google Scholar]
- 56.Metcalf MG. Incidence of ovulation from the menarche to the menopause: observations of 622 New Zealand women. N Z Med J. 1983;96(738):645–648. [PubMed] [Google Scholar]
- 57.de Zambotti M, Cellini N, Goldstone A, Colrain IM, Baker FC. wearable sleep technology in clinical and research settings. Med Sci Sports Exerc. 2019;51(7):1538–1557. doi: 10.1249/MSS.0000000000001947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Colvonen PJ, DeYoung PN, Bosompra NA, Owens RL. Limiting racial disparities and bias for wearable devices in health science research. Sleep. 2020;43(10). doi: 10.1093/sleep/zsaa159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grandner MA, Williams NJ, Knutson KL, Roberts D, Jean-Louis G. Sleep disparity, race/ ethnicity, and socioeconomic position. Sleep Med. 2016;18:7–18. doi: 10.1016/j.sleep.2015.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pengo MF, Won CH, Bourjeily G. Sleep in women across the life span. Chest. 2018;154(1):196–206. doi: 10.1016/j.chest.2018.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baker FC, de Zambotti M, Colrain IM, Bei B. Sleep problems during the menopausal transition: prevalence, impact, and management challenges. Nat Sci Sleep. 2018;10:73–95. doi: 10.2147/NSS.S125807 [DOI] [PMC free article] [PubMed] [Google Scholar]