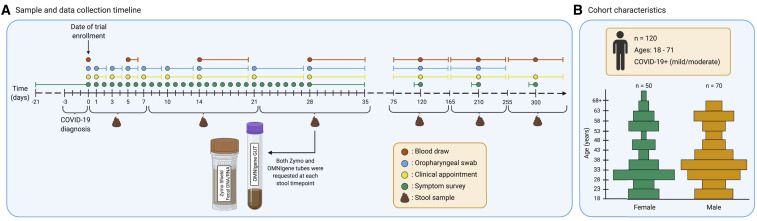
Figure 1.
Summary of study protocol and cohort demographics
(A) Sample and data collection timeline represented in days. Day 0 marks the day of enrollment in the trial, within 72 h of a COVID-19 diagnosis. Each sample collection event is marked by a colored dot, where orange represents a blood draw and blue an oropharyngeal (OP) swab. Additionally, clinical appointments and symptom surveys are marked by yellow and green dots, respectively. Some of these events are marked by day ranges to represent collection time frames. The symptom survey at day 0 retrospectively collected symptomatology for 3 weeks prior to enrollment using a single questionnaire. Symptom surveys at time points centered around days 120, 210, and 300 retrospectively collected symptomatology for 1 week prior to the appointment using a single questionnaire at each timepoint. Collection of stool samples and their respective day ranges are marked below the timeline. Subjects were asked to provide samples in the OMNIgene GUT collection tube (OG) and the Zymo DNA/RNA shield fecal collection tube (ZY) at six time points.
(B) Cohort characteristics. 120 participants were enrolled in the clinical trial. Participants had a COVID-19 infection of mild to moderate severity and were between the ages of 18 and 71. The age and sex distributions of the paticipants are reprented here. The x axis separates the groups by self-reported sex, and the y axis lists age in years. Each bar represents a range of 5 years.