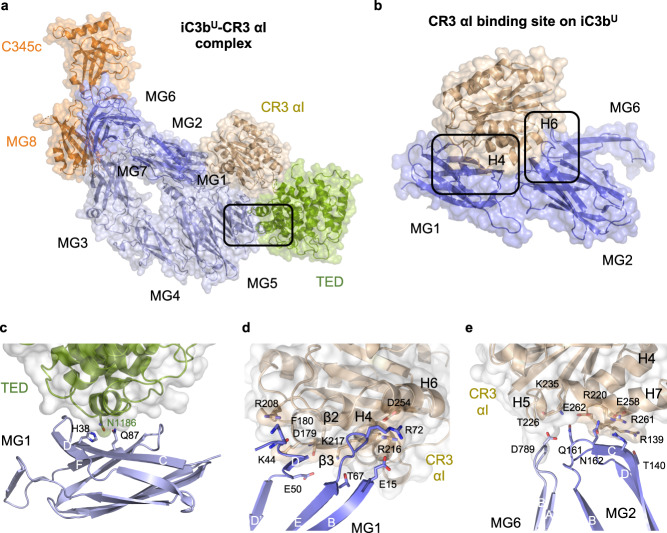
Fig. 3. CR3 αI binding site and interactions with the C3c moiety and the TED domain in iC3bU–CR3 αI.
a Overall structure of the iC3bU–CR3 αI complex in the upright orientation characteristic of the conformation. Structures are shown in cartoon representation and overlaid by a semi-transparent molecular surface. iC3bU is color-coded as in Fig. 1. CR3 αI is shown in wheat color. The region inside the black outline is shown in (c). b Interaction of CR3 αI with the MG domains MG1–MG2. The boxed areas are magnified in panels (d) and (e). c Interface between the TED and MG1 domains. d Close-up of the interaction of CR3 αI with the MG1 domain. e Close-up of the interaction of CR3 αI with the MG2 domain. The tip of the turn between β-strands βA and βB of MG6 provides an additional contact. Amino acid residues which establish hydrogen bond or salt bridge interactions across the interfaces shown in panels (c–d) are shown as sticks.