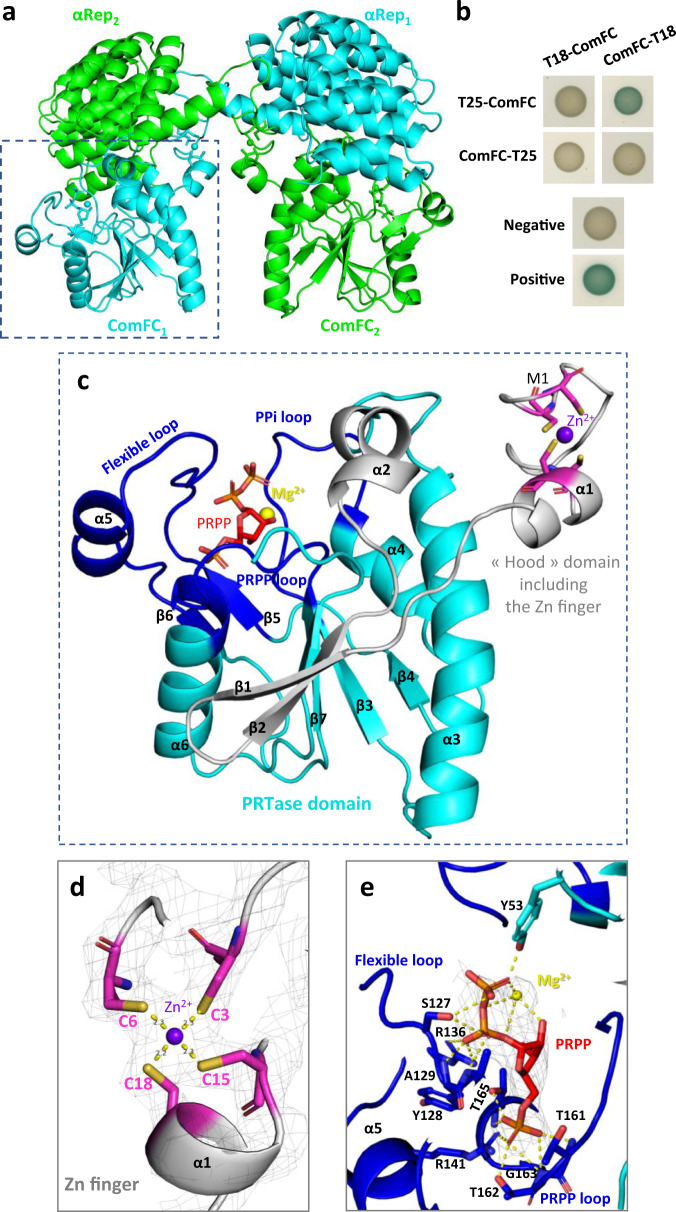
Fig. 5. H. pylori ComFC harbours a PRT and Zn-finger domains.
a Crystal structure of the αRep-ComFC domain-swapped dimer. The two protein fusions are in green and blue. The αRep was evolved against ComFC to develop a specific interaction surface. In the crystal packing, each αRep returned to the ComFC of another fusion, allowing the crystallisation of an artificial dimer. The PRPP co-crystallised with the protein is represented by sticks, and the Zn2+ ion is schematized by a sphere. b Bacterial two‐hybrid assay of H. pylori ComFC. Representative images of reporter strains grown on plates supplemented with IPTG and X-Gal are shown. c ComFC structure. The three loops characteristic of the PRTase fold are in dark blue and the “hood” domain is in cyan. The PRPP is in red sticks and the Zn2+ and Mg2+ ions are represented by purple and yellow spheres, respectively. d 2Fo-Fc electron density map (grey mesh, contoured at 1.5 sigma) of the 4 cysteines, coloured as in c, forming 4 coordination bonds (yellow dotted lines) with the Zn2+ ion. e 2Fo-Fc electron density omit-map (grey mesh, contoured at 1.5 sigma) of the PRPP and the Mg2+ ion in interaction with ComFC (coloured as in c). The shared hydrogen bonds between ComFC amino acids (in dark blue sticks) and PRPP or Mg2+ are shown as yellow dotted lines.