Abstract
High mammographic density and exposure to sex steroids are independent risk factors for breast cancer by yet unknown mechanisms. Inflammation is one hallmark of cancer and the tumor necrosis factor family of proteins (TNFSFs) and receptors (TNFRSFs) are key determinants of tissue inflammation. The relationship between TNFSFs/TNFRSFs and breast tissue density or local breast estradiol levels is unknown. We investigated whether TNFSFs and soluble TNFRSFs (sTNFRSFs) are dysregulated in vivo in human breast cancer and dense breast tissue of postmenopausal women. We explored TNFSF/TNFRSF correlations with breast density and estradiol, both locally in the breast and in abdominal subcutaneous (s.c.) fat as a measure of systemic effects. Microdialysis was used for local sampling of in vivo proteins and estradiol in a total of 73 women; 12 with breast cancer, 42 healthy postmenopausal women with different breast densities, and 19 healthy premenopausal women. Breast density was determined as lean tissue fraction (LTF) using magnetic resonance imaging. Microdialysis was also performed in estrogen receptor (ER) positive breast cancer in mice treated with the pure anti-estrogen fulvestrant and tumor tissue was subjected to immunohistochemistry. 23 members of the TNFSF/sTNFRSF families were quantified using proximity extension assay.Our data revealed upregulation of TNFSF10, 13 and 13B, TNFRSF6, 6B, 9, 11A, 11B, 13B, 14, and 19, and TNFR-1 and -2 in ER+ breast cancer in women. In dense breast tissue TNFSF10, 13, and 14, TNFRSF3, 6, 9, 10B, 13B, 14, 19, and TNFR-1 and -2 were upregulated. Certain TNFSFs/TNFRSFs were increased in premenopausal breasts relative to postmenopausal breasts. Furthermore, estradiol correlated with most of the TNFSF/sTNFRSF members, though LTF only correlated with some of the proteins. Several of these associations were breast tissue-specific, as very few correlated with estradiol in abdominal s.c. fat. Estrogen dependent regulations of TNFSF2 (TNF-α) and TNF-R2 were corroborated in ER+ breast cancer in mice. Taken together, our data indicate TNFSFs/sTNFRSFs may represent potential targetable pathways for treatment of breast cancer patients and in prevention of breast cancer development in women with dense breasts.
Keywords: mammography, microdialysis, mammary gland, breast cancer, sex steroids, estradiol
Introduction
Dense breast tissue, as depicted on mammography, is a major independent risk factor for breast cancer; women with dense breasts have a 4-6-fold increased risk of the disease as compared to women with nondense breasts (1, 2). An inversed association of the absolute amount of nondense area and risk of breast cancer has also been shown (2). It has been suggested that 30% of all breast cancer cases occur in women with > 50% dense area (1, 3). Despite these associations, the biological mechanisms underlying the increased risk are poorly understood and no preventive therapy to these women is available. The major histological difference between normal breast tissue of varying density is higher amounts of stroma, including collagen, in dense breasts and higher amounts of fat in nondense breasts (4, 5). Approximately 5-10% of the tissue area in normal breasts comprise epithelial cells and there are no conclusive data on differences between dense and nondense breast regarding the quantity or proliferation rate of these cells (4–8). Thus, the microenvironment surrounding the epithelial cells is a key determinant in breast cancer initiation and progression. Exposure to sex steroids including estrogens is an established risk factor for breast cancer (9, 10). However, no association between circulating estrogen levels and breast density has been determined (1).
Adipose tissue is a major component in breast tissue and adipocytes, in concert with immune cells, are major contributors to tumor necrosis factor (TNF) production and secretion. TNF-α is a member of the TNF superfamily (TNFSF) with number 2 of this family, TNFSF2. TNFSFs binds to proteins in the TNF receptor superfamily (TNFRSF) (11). TNFSFs/TNFRSFs act as local regulators in a paracrine/autocrine fashion and the biological activity reflects the balance between these regulators in the microenvironment. Consequently, the local regulation of the TNF system is complex and its role in cancer is controversial, and both tumor-promoting and tumor-inhibiting actions have been detected. However, TNFSF2 has been shown to be a key player in tumor-stroma inflammation in breast cancer (12). As these proteins are released and cleaved locally in the tumor microenvironment into soluble proteins, mRNA analysis or immunohistochemistry cannot detect, quantify, or reveal the intricate and complex interplay that occurs in the extracellular tissue microenvironment. How TNFSFs/TNFRSFs are dysregulated in breast cancer or affected by tissue density in normal breasts is not fully understood and the role of estradiol in their regulation is today undetermined.
Here we provide novel data showing that the majority of TNFSF and sTNFRSF were significantly dysregulated in human breast cancer. In healthy postmenopausal women, normal dense breast tissue compared to normal nondense breasts, exhibited similar dysregulation as breast cancers. Additionally, premenopausal breast tissue displayed increased levels of several of these proteins as compared to postmenopausal breasts. Furthermore, we show that breast density, determined with a continuous measure density using on magnetic resonance imaging (MRI), correlated with some of the proteins, whereas estradiol correlated with the majority of the TNFSF/sTNFRSF family members. Additionally, several of these associations were breast tissue specific as only a minority of the proteins correlated with estradiol in abdominal subcutaneous fat (s.c.). Thus, estradiol seems to play a prominent role for the local control of TNFSF/sTNFRSF family members in normal human breast tissue in vivo. Our results identify the TNFSF/sTNFRSF family as potential targets for breast cancer therapeutics and for prevention measures to postmenopausal women with dense breasts and for estrogen dependent breast cancer progression.
Materials and Methods
Subjects
The study was carried out in accordance with the Declaration of Helsinki and the Regional Ethical Review Board of Linköping, Sweden approved the study. All subjects gave informed consent. A total of 73 women were included in the study. Of these 12 women with estrogen receptor positive breast cancer were investigated with microdialysis before surgery.
Forty-two healthy postmenopausal women (ages 55–74 years) were consecutively recruited for the study as previously described (13). In brief, on the mammography screening facility at Linköping University Hospital, mammograms of postmenopausal women were categorized according to the Breast Imaging Reporting and Data System (BI-RADS) (14). Women with either entirely fatty nondense (BI-RADS A) or extremely dense (BI-RADS D) were invited to the study. After informed consent, included women underwent magnetic resonance imaging (MRI) for calculations of lean tissue fraction (LTF) as a continuous measure of breast density, as previously described (13). LTF was calculated in a volume selection of 30 x 30 x 30 mm within the glandular tissue in the upper lateral quadrant of the left breast. After a second review of the mammograms two women had been miscategorized and were not dense or nondense. These two women were not included in the analyses.
Additionally, 19 nulliparous premenopausal women (ages 20–32 years) with a history of regular menstrual cycles (cycle length, 27–34 days) were included. None of the healthy volunteer women had a history of breast cancer or were currently using (or had used within the past 3 months) hormone replacement therapy, sex steroid-containing contraceptives, anti-estrogen therapies, including selective estrogen receptor modulators, or degraders.
Microdialysis Procedure
Prior to insertion of the microdialysis catheters 0.5 mL lidocaine (10 mg/mL) was administrated intracutaneously. Microdialysis catheters (M Dialysis AB, Stockholm, Sweden), which consisted of a tubular dialysis membrane (diameter 0.52mm, 100,000 atomic mass cut-off) glued to the end of a double-lumen tube were inserted via a splitable introducer (M Dialysis AB), connected to a microinfusion pump (M Dialysis AB) and perfused with 154 mmol/L NaCl and 60g/L hydroxyethyl starch (Voluven®; Fresenius Kabi, Uppsala, Sweden), at 0.5 µL/min. The women with ongoing breast cancer were investigated with 10 mm long membranes; one catheter was inserted within the cancer tissue and the other into normal adjacent breast tissue. The healthy volunteer women were investigated with 20-mm long microdialysis membranes; one was placed in the upper lateral quadrant of the left breast and directed towards the nipple and the other in abdominal s.c. fat as previously described (15–25). The premenopausal women were subjected to microdialysis in the luteal phase of the menstrual cycle. The microdialysis catheter was placed in the same quadrant where LTF was determined.
After a 60-min equilibration period, the outgoing perfusate was stored at -80°C for subsequent analysis.
Breast Cancer Model
The Institutional Animal Ethics Committee at Linköping University approved this study, which conformed to regulatory standards of animal care. Oophorectomized athymic mice (Balb/C-nu/nu, 6-8 weeks old, Scanbur, Sweden) were housed at Linköping University in ventilated cages with a light/dark cycle of 12/12 hours with rodent chow and water available ad libitum. Mice were anesthetized via intraperitoneal (i.p.) injection of ketamine/xylazine and implanted with a subcutaneous (s.c.) 3-mm pellet containing either 17β-estradiol (0.18 mg/60-day release, Innovative Research of America, Sarasota, FL, USA) or placebo. The active pellet releases serum concentrations of 150-250 pM estradiol (26). 5 × 106 MCF-7 cells were injected into the dorsal mammary fat pads in 200 µl PBS. MCF-7 cells require estrogen for tumor formation and growth in mice, therefore, a non-estrogen control group is not possible to achieve. When tumors reached ≈20 mm2 in size the mice were treated with fulvestrant (5 mg/mouse twice per week, s.c.) in addition to the estradiol exposure.
Microdialysis in Mice
Tumor-bearing mice with size-matched tumors were anesthetized with i.p. injections of ketamine/xylazine and maintained by repeated s.c. injections of ketamine/xylazine. Body temperatures were maintained using a heat lamp. Microdialysis probes with 4-mm membranes (CMA 20, 100-kDa cutoff; CMA Microdialysis AB, Kista, Sweden) were inserted into tumor tissue and connected to a microdialysis pump (CMA 102; CMA Microdialysis AB) perfused at 0.6 μl/min with 154 mmol/L NaCl and 60g/L hydroxyethyl starch (Voluven®; Fresenius Kabi, Uppsala, Sweden), as previously described (27, 28). After a 60-minute equilibrium period, outgoing perfusates (i.e., microdialysates) were collected and stored at -80°C for subsequent analysis.
Histology
Formalin-fixed tumors from tumor-bearing mice were paraffin-embedded and cut in 4-μm sections, de-paraffinized, and exposed to rabbit anti human TNF Receptor I antibody (Abcam Cat# ab19139, RRID : AB_2204128), mouse anti human TNF Receptor II antibody (Abcam Cat# ab8161, RRID : AB_306318), Dako EnVision+System-HRP Labelled Polymer anti-rabbit (Dako Cat# K4002) and anti-mouse (Dako Cat# K4000). Mayer’s hematoxylin was used for counterstaining. Negative controls, exposed to the labelled polymers only, showed no staining.
Images of 10 areas of each tumor section from 3 mice each treatment group were acquired on an Olympus BX43 microscope ×40/0.75 magnification. For collagen content Trichrome stain kit (Abcam, Cat# ab 150686) was used according to the manufacturer’s protocol.
Protein Quantifications
The microdialysis samples were analyzed using a multiplex proximity extension assay (PEA, Olink Bioscience, Uppsala Sweden) as previously described (29–31). In brief, 1 μL sample was incubated with proximity antibody pairs tagged with DNA reporter molecules. The DNA tails formed an amplicon by proximity extension, which was quantified by high-throughput real-time PCR (BioMark™ HD System; Fluidigm Corporation, South San Francisco, CA, USA). The generated fluorescent signal correlate with protein abundance by quantitation cycles (Cq) produced by the BioMark Real-Time PCR Software. To minimize variation within and between runs, the data were normalized using both an internal control (extension control) and an interplate control and transformed using a predetermined correction factor. The pre-processed data were provided in the arbitrary unit normalized protein expression (NPX) on a log2 scale, which were then linearized by using the formula 2NPX. A high NPX value corresponded to a high protein concentration. Values represented a relative quantification meaning that no comparison of absolute levels between different proteins could be made.
Estradiol Analysis
Estradiol levels in the microdialysis samples were analyzed using a high sensitivity immunoassay kit (DRG International, Springfield Township, NJ, USA).
Statistical Analyses
Statistical analyses were performed using nonparametric Wilcoxon matched-pairs signed rank tests or Kruskal Wallis tests followed by unpaired Mann-Whitney U tests when more than two groups were compared as the data was non-normally distributed. Spearman’s correlation test was used for calculations of correlations. A P<0.05 was considered statistically significant. Statistics were performed with Prism 9.0 (GraphPad, San Diego, CA, USA).
Results
TNFSF/sTNFRSF Family of Proteins in Human Breast Cancer
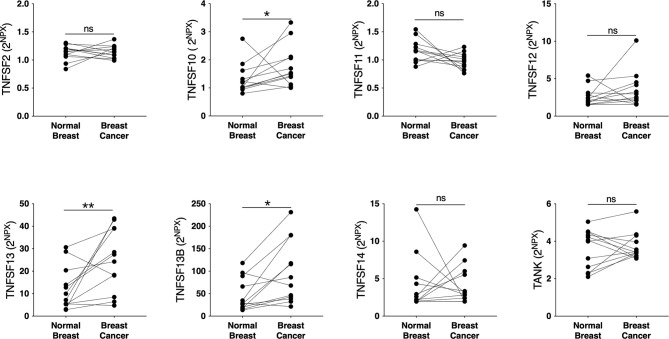
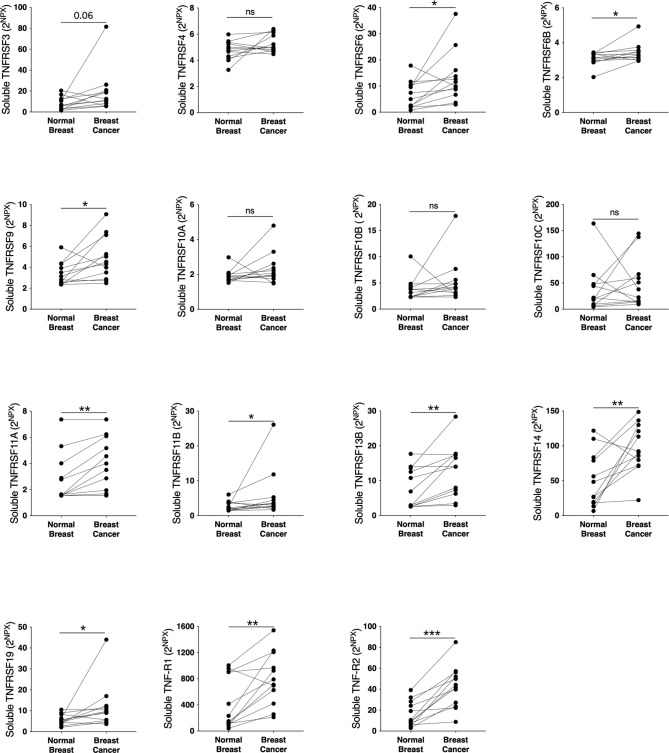
To elucidate whether the TNFSF/sTNFRSF family of proteins were affected, and therefore possible drug targets, in human breast cancer we performed microdialysis in women with breast cancer prior to their surgery to retrieve extracellular molecules in live tissues. The women with breast cancer were included regardless of their breast density, which was undetermined. As shown in Figure 1 , TNFSF10, -13 and -13B were upregulated whereas TNFSF2, -12, -14, and TANK were unaffected in breast cancer compared to normal adjacent breast tissue. Regarding the soluble receptors the levels of sTNFRSF6, -6B, -9, -11A, -11B, -13B, -14, -19 and sTNF-R1 and -R2 were significantly increased in breast cancers whereas sTNFRSF4, -10A, -10B and -10C were unaffected, Figure 2 . Borderline significance of sTNFRSF3 (p=0.06) were demonstrated, Figure 2 .
Figure 1.
Extracellular levels of TNFSF in vivo in breast cancers and adjacent normal breast tissue. 12 patients with estrogen receptor positive breast cancer underwent microdialysis before surgery. One catheter was inserted into the breast cancer and another into adjacent normal breast tissue. Proteins were quantified using proximity extension assay. Data represents protein abundance in linear values (2NPX as described in the materials and methods section). *P < 0.05, **P < 0.01, ns, not significant.
Figure 2.
Extracellular levels of soluble TNFRSF in vivo in breast cancers and adjacent normal breast tissue. 12 patients with estrogen receptor positive breast cancer underwent microdialysis before surgery. One catheter was inserted into the breast cancer and another into adjacent normal breast tissue. Proteins were quantified using proximity extension assay. Data represents protein abundance in linear values (2NPX as described in the materials and methods section). *P < 0.05, **P < 0.01, ***P < 0.001, ns, not significant.
TNFSF/sTNFRSF Family of Proteins in Normal Human Breast Tissues
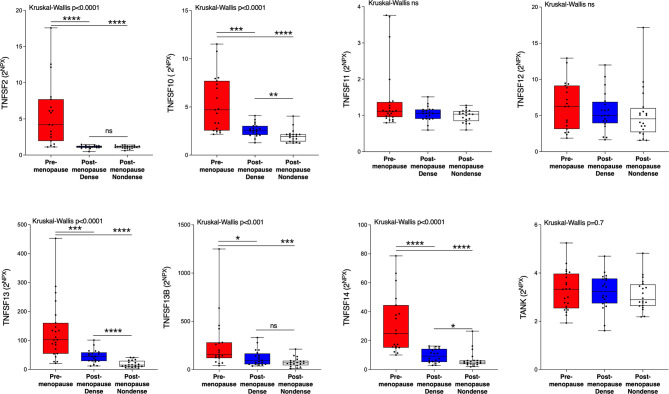
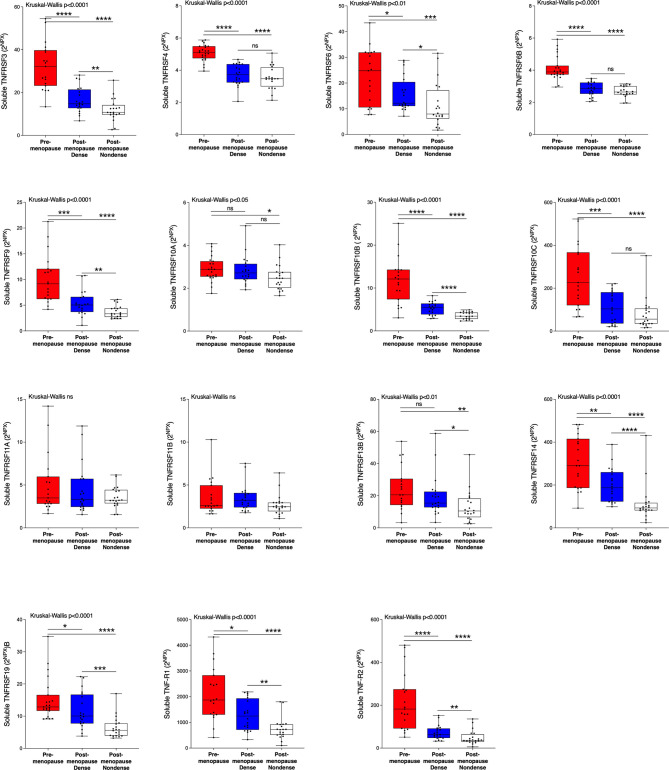
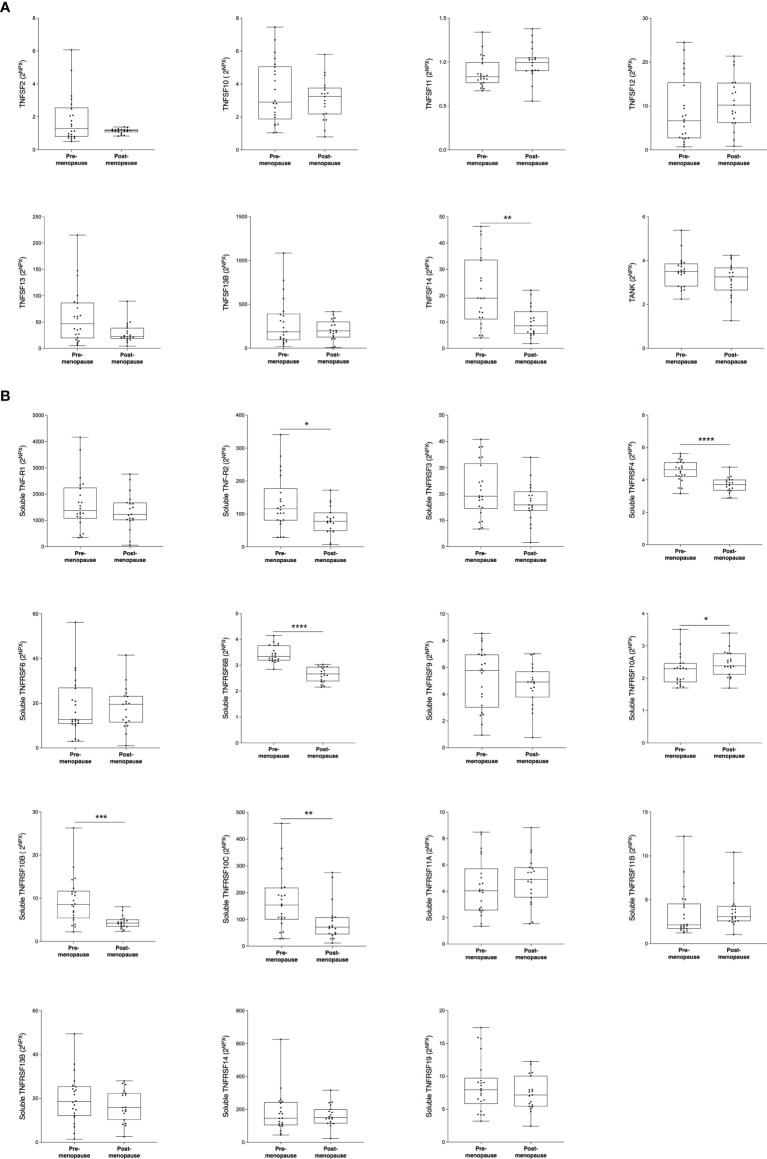
Next, we wanted to investigate whether the TNFSF/sTNFRSF proteins were affected in normal breast tissue with inherently high risk of breast cancer, dense breast tissue of postmenopausal women, as compared to nondense breasts with inherently low risk of breast cancer. Additionally, to be able to investigate whether estradiol affects these proteins a group of premenopausal women were included. As shown in Figure 3 , 5 ligands, TNSFS2, -10, -13, -13B and 14, were significantly increased in premenopausal breasts as compared to both dense and nondense breasts in postmenopausal women. Furthermore, TNFSF10, -13 and -14 were increased in dense breasts compared to nondense breasts in postmenopausal women, Figure 3 . No significant changes were detected of TNFSF11, -12 and TANK, Figure 3 . Regarding the soluble receptors, 11 were increased in premenopausal breast tissue compared to both dense and nondense postmenopausal breasts; sTNFRSF3, -4, -6, -6B, -9, -10B, -10C, -14, -19, and TNF-R1 and R2, Figure 4 . Furthermore, sTNFRSF3, -6, -9, -10B, -13B, -14, -19, and TNF-R1 and R2 were increased in dense breasts compared to nondense breasts, Figure 4 . No significant changes were detected of sTNFRSF11A and 11B, Figure 4 .
Figure 3.
Extracellular levels of TNFSF in vivo in healthy normal breast tissue of premenopausal women and postmenopausal women with different breast densities. A total of 59 women underwent microdialysis of their left breast; 40 postmenopausal healthy volunteer women, attending the regular mammography-screening program categorized as either having dense (n = 20, blue boxes) or nondense breasts (n = 20, clear boxes) and 19 premenopausal women (red boxes). Proteins were quantified using proximity extension assay. Data represents extracellular local protein abundance in linear values (2NPX as described in the Methods section). A significant Kruskal-Wallis was followed by Mann Whitney U-test. Data are displayed as box plots with median and 10–90 percentile. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, ns=not significant.
Figure 4.
Extracellular levels of soluble TNFRSF in vivo in healthy normal breast tissue of premenopausal women and postmenopausal women with different breast densities. A total of 59 women underwent microdialysis of their left breast; 40 postmenopausal healthy volunteer women, attending the regular mammography-screening program categorized as either having dense (n = 20, blue boxes) or nondense breasts (n = 20, clear boxes) and 19 premenopausal women (red boxes). Proteins were quantified using proximity extension assay. Data represents extracellular local protein abundance in linear values (2NPX as described in the Methods section). A significant Kruskal-Wallis was followed by Mann Whitney U-test. Data are displayed as box plots with median and 10–90 percentile. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, ns, not significant.
Correlations Between TNFSF/sTNFRSF Proteins With Estradiol and LTF Locally in Breast Tissue
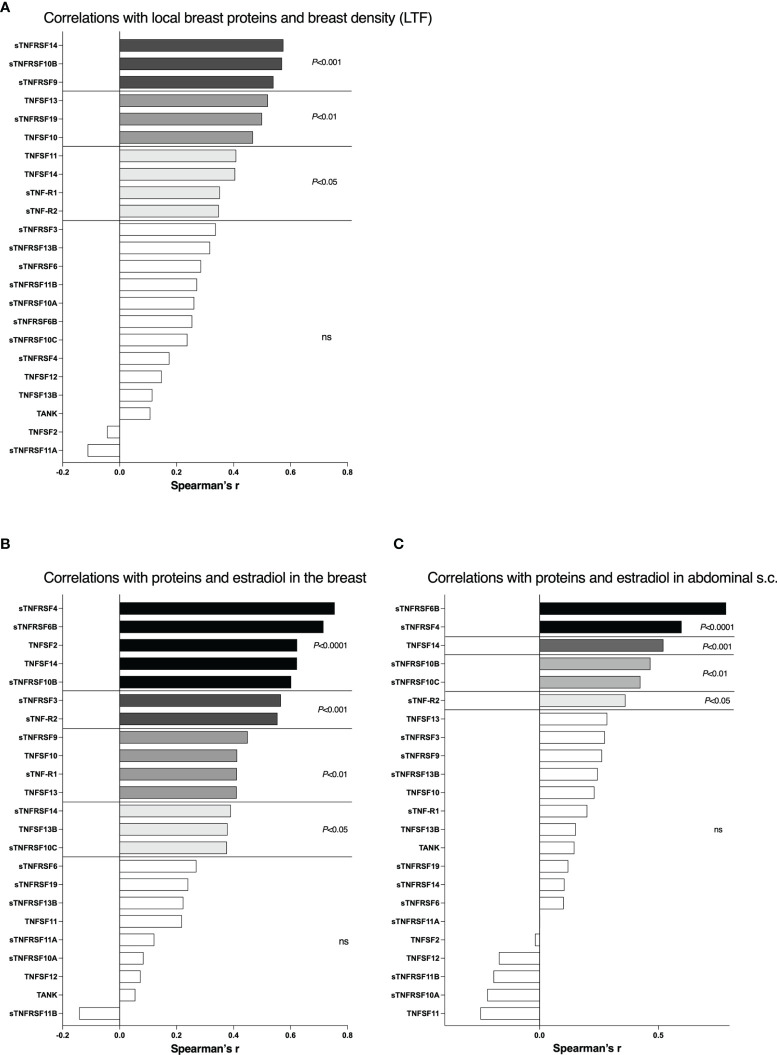
There were no differences in local breast estradiol levels in the postmenopausal women with nondense or dense breast tissue, 36 (16-69) pmol/l in nondense vs. 38 (14-74) pmol/l in dense breasts (median (range)). As the estradiol levels were similar in both postmenopausal groups correlations with breast density measured by LTF and proteins in TNFSF/sTNFRSF family was possible to perform. As shown in Figure 5A , 10 of the 23 proteins correlated significantly with LTF whereof sTNRSF14, -10B, and -9 being the most significant, p<0.001.
Figure 5.
Correlations between local breast levels of soluble TNFRSF and TNFSF with breast density and local breast estradiol levels. A total 40 postmenopausal healthy volunteer women, attending the regular mammography-screening program and 19 healthy premenopausal women underwent microdialysis of their left breast for sampling of extracellular proteins in vivo. Proteins and estradiol (E2) were quantified in the microdialysate as described in the materials and methods section. Breast density was quantified as lean tissue fraction (LTF) determined in the MRIs. (A) Correlations of local breast levels of TNFSFs/sTNFRSFs and LTF in postmenopausal women. (B) Correlations of local breast levels of TNFSFs/sTNFRSFs and local breast estradiol levels. Premenopausal women and postmenopausal women with dense breasts were included. (C) Correlations of local levels of TNFSFs/sTNFRSFs and local estradiol levels in abdominal s.c. fat. Bars represent Spearman’s Rank correlation coefficient. White bars, not significant.
The premenopausal women, which per definition have dense breasts, exhibited significantly increased local breast estradiol levels, 205 (73-264) pmol/l, compared to postmenopausal women with dense breasts. Thus, in these two groups correlations depending on estradiol were possible to perform as both groups represent dense breast tissue. 14 of the 23 proteins correlated significantly with estradiol whereof sTNFRSF4, -6B, TNF, TNFSF14 and sTNFRSF10B being highly significant, p<0.0001, Figure 5B . As a measure of systemic effects, micodialysis was used to collect extracellular factors from abdominal s.c. fat in all healthy volunteers. By this approach breast tissue specific alterations can be deciphered. As shown in Figure 5C , 6 of the proteins correlated significantly with local fat estradiol whereof sTNFRSF6B and sTNFRSF4 being highly significant, p<0.0001.
TNFSF/sTNFRSF Proteins in Abdominal s.c. Fat
The specific data from s.c. abdominal fat were as follows; local estradiol levels were 36 (27-60) pmol/l in postmenopausal women vs. 202 (88-270) pmol/l in premenopausal women (median (range)), p<0.0001. As shown in Figure 6A among the ligands only TNFSF14 was significantly increased in premenopausal women. Among the soluble receptor shown in Figure 6B , sTNF-R2, sTNFRSF4, -6B, 10A, 10B, and 10C were significantly increased in premenopausal women.
Figure 6.
Local levels of TNFSF/sTNFRSF in abdominal s.c. fat. The women described in Figure 5 were subjected to microdialysis of the abdominal s.c. fat at the same time point as the microdialysis of breast tissue. Proteins were quantified in the microdialysate as described in the materials and methods section. Mann Whitney U-test was used. Data are displayed as box plots with median and 10–90 percentile. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. (A) TNF ligands (TNFSFs) (B) Soluble TNF receptors (sTNFRSFs).
TNFSF2-TNFR in Experimental Breast Cancer
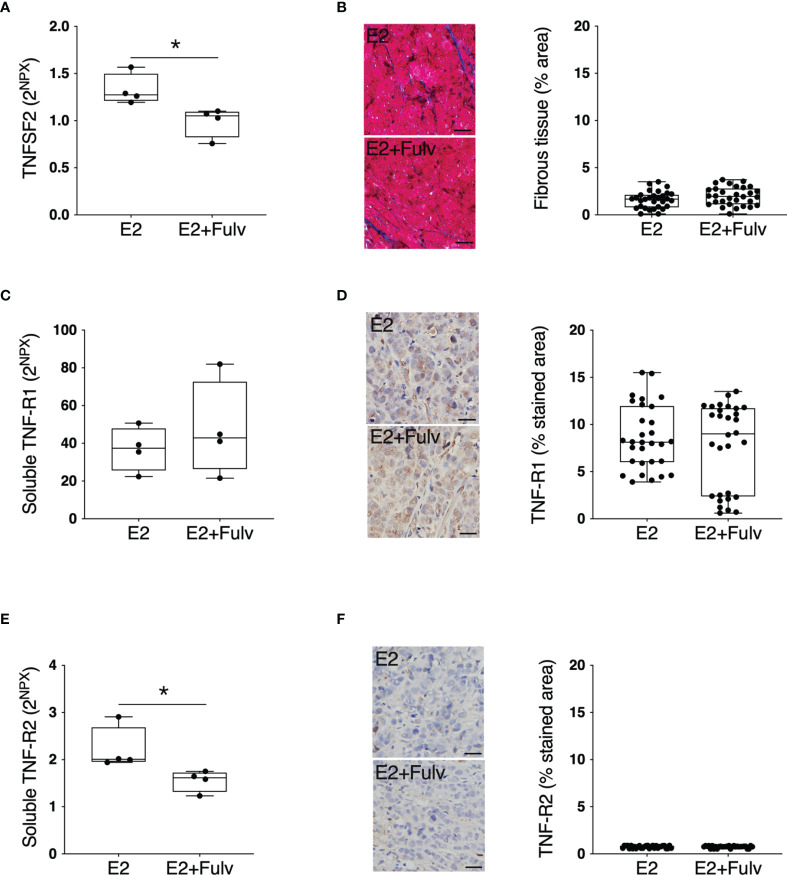
Next, we set up experimental estrogen receptor positive (ER+) breast cancer in mice to investigate possible estrogen regulation of extracellular TNFSF2 and its main receptors TNF-R1 and TNF-R2 in cancerous tissue. As shown in Figure 7A treatment with the pure anti-estrogen fulvestrant significantly decreased the in situ levels of extracellular TNFSF2. The tumors comprised very little stroma with no differences between the groups, Figure 7B . No differences were found in soluble or cellular TNF-R1, Figures 7C, D . Additionally, soluble TNF-R2 was significantly decreased by fulvestrant therapy whereas no significant difference was found of cellular TNF-R2, Figures 7E, F .
Figure 7.
TNFSF2, TNF-R1, and TNF-R2 in experimental ER+ breast cancer. Oophorectomized athymic mice supplemented with physiological levels of estradiol (E2) were injected with MCF-7 into the dorsal mammary fat pads. At similar tumor sizes, mice either continued with E2 or were additionally treated with fulvestrant (E2+Fulv) (5 mg/mouse every 3 days, s.c.). Size-matched tumors from the different treatment groups underwent microdialysis for sampling of extracellular proteins in vivo, which were quantified using proximity extension assay. Data represents extracellular local protein abundance in linear values (2NPX as described in the Methods section). Tumor sections were subjected to immunohistochemistry. Data are presented as the mean ± SD. *P < 0.05. (A) Extracellular TNFSF2, n = 4 animals per group. (B) Tumor sections from each treatment group were stained for collagen (blue) and quantified as the percentage of area with positive staining. Representative sections are depicted. Scale bar = 20 µm. (C) Soluble TNF-R1, n = 4 animals per group. (D) Tumor sections from each treatment group were stained for TNF-R1 and quantified as the percentage of area with positive staining. Representative sections are depicted. Scale bar = 20 µm. (E) Soluble TNF-R2, n = 4 animals per group. (F) Tumor sections from each treatment group were stained for TNF-R2 and quantified as the percentage of area with positive staining. Representative sections are depicted. Scale bar = 20 µm.
Discussion
To the best of our knowledge no previous data on soluble members of the TNFSF/TNFRSF family of proteins in breast cancer and normal breast tissue with high risk of breast cancer has been studied. As summarized in Table 1 , we demonstrate that most of these proteins were significantly increased in human breast cancers compared to normal adjacent breast tissue. In postmenopausal women dense breasts, as compared to nondense breasts, exhibited similar alterations as breast cancers. Additionally, breast tissue in premenopausal women exhibited significant different levels of many of the TNFSF/TNFRSF proteins compared to both breast tissue types of postmenopausal women. Our data revealed that breast density correlated significantly with 10 of the 23 proteins whereas estradiol exhibited significant positive correlations with 14. An estrogen dependent regulation of soluble extracellular TNF and TNF-R2 was corroborated in experimental ER+ breast cancer in mice.
Table 1.
Microdialysis was performed in breast tissue in women and proteins and estradiol were quantified in the dialysates.
| Protein | Breast cancer vs. normal breast | Dense vs. nondense breast tissue | Correlation with local breast estradiol | Correlation with local breast LTF |
|---|---|---|---|---|
| TNFSF2 | Yes | |||
| TNFSF10 |

|

|
Yes | Yes |
| TNFSF11 | Yes | |||
| TNFSF12 | ||||
| TNFSF13 |

|

|
Yes | Yes |
| TNFSF13B |

|
Yes | ||
| TNFSF14 |

|
Yes | Yes | |
| TANK | ||||
| sTNFRSF3 |

|
Yes | ||
| sTNFRSF4 | Yes | |||
| sTNFRSF6 |

|

|
||
| sTNFRSF6B |

|
Yes | ||
| sTNFRSF9 |

|

|
Yes | Yes |
| sTNFRSF10A | ||||
| sTNFRSF10B |

|
Yes | Yes | |
| sTNFRSF10C | Yes | |||
| sTNFRSF11A |

|
|||
| sTNFRSF11B |

|
|||
| sTNFRSF13B |

|

|
||
| sTNFRSF14 |

|

|
Yes | Yes |
| sTNFRSF19 |

|

|
Yes | |
| sTNFR-1 |

|

|
Yes | Yes |
| sTNF-R2 |

|

|
Yes | Yes |
Magnetic resonance imaging was used for quantifications of lean tissue fraction (LTF), a continuous measure of breast density.
TNFSF/TNFRSF families comprise 19 ligands that can bind to one or more of 29 structurally similar receptor proteins (32, 33). These proteins play an important role in various biological processes and aberrant production and receptor signaling by TNFSF/TNFRSF has been associated with the pathogenesis of several inflammatory diseases and cancer (11). Most members of the TNFRSF contain transmembrane (TM) domains and by proteolytic processing of these, soluble ligand-binding molecules from the receptors are formed, sTNFRSF. Local accumulation of the soluble forms of receptors and ligands will interfere with the binding of membrane-bound forms and act both as inhibitors and buffering agents, which may lead to decreased intensity of signaling activation or an extended duration of signaling. TNF signaling can also be modulated by binding of TNFs to decoy receptors such TNFRSF6B, TNFRSF10C, TNFRSF10D, and TNFRSF11B (11).
Due to the initial observations that TNF could cause tumor necrosis TNF therapy was tested in clinical trials of cancer. Due to systemic toxicity these were, however, terminated and TNF is now only approved in a loco-regional setting as a cancer therapeutic (34, 35). Later studies have revealed that the TNF signaling is complex and somewhat contradictory. In preclinical models of breast cancer, TNF signaling may promote migration and invasion of breast cancer cells, as well as to induce apoptosis and exert cytotoxic effects in vitro (34). For example, when TNFSF2 binds to TNF-R1 and TNF-R2 apoptosis may be stimulated but both receptors have also been shown to activate proliferation by the NF-kB pathway (34). Additionally, TNFSF6 may be proapoptotic when binding to TNFRSF6 by activating caspase 8 complex whereas apoptosis is inhibited by its binding to TNFRSF6B (36). TNFRSF6B itself can attenuate inflammation and overexpression of this proteins has been related to breast cancer progression (37, 38). TNFSF10 is another ligand with both pro- and anti-apoptotic effects as well as effects on the immune response (39). These data have been corroborated by genome wide screens that recently uncovered the TNF-pathway as essential for susceptibility of immunotherapy and important for the understanding of the immune response in cancer (40). Specifically, TNFSF13 and TNFSF14 can increase the effects of immunotherapy by facilitating immune cell mobilization into the cancer (41–43). As recently reviewed, several members of TNFSF/TNFRSF families are key for the coordination of mechanism that result in activation or inhibition of the immune response in both inflammatory diseases as well as in cancer (33). Most members of the TNFRSF family are involved in apoptosis regulation and activation of inflammation via NF-κB activation; TNFRSF3 and 6 may induce apoptosis as well as activate the immune response by increasing secretion of inflammatory cytokines including IL-8 as well as induce apoptosis of tumor cells (44, 45). TNFRSF9 increase inflammation, affect the T-cell population as well as increase proliferation of peripheral monocytes (46). Similar to other members of the TNFRSF family, TNFRSF10B and 10C can induce inflammation and may either enhance or inhibit apoptosis depending on the expression of other cytokines in the microenvironment (47). TNFRSF14 is expressed on a broad range of cancer cells and may activate the inflammatory response, which has implicated its role in autoimmune pathogenesis (48). TNFRSF19 may be involved in beta-catenin regulation of NF-κB and has primarily been associated with melanoma, glioblastoma, and colorectal cancer (49, 50). However, further studies of the specific roles of the different TNFSFs/TNFRSFs in mammographic density or breast cancer progression need to be performed.
The role of estrogen in the regulation of TNFSF2 is somewhat controversial and there is no consensus in the literature. In bone tissue, estradiol has been shown to downregulate TNFSF2 (51). Contrary to this, it has been demonstrated that estradiol up-regulates TNFSF2 in dendritic cells in mice and in synovial tissue of humans (52, 53). Additionally, anti-estrogen decreases the levels of TNFSF2 in experimental models of breast cancer (54). Our data revealed a significant positive correlation between estradiol and TNFSF2 determined locally in normal breast tissue supporting an estrogen dependent regulation in the mammary gland. The data from normal breast tissue was corroborated by our results from experimental ER+ breast cancer where treatment with the pure anti-estrogen fulvestrant significantly decreased the levels of extracellular in vivo TNF. Surprisingly, in ER+ human breast cancers no increased levels, as compared to normal adjacent breast tissues, of TNFSF2 were detected. However, all samples from the breast cancer patients were in the lower range of the detection limit of the assay for TNFSF2 and may therefore not accurately reflect possible differences. Increased sensitivity of the assay may yield different results and this needs to be performed before any conclusions can be made regarding extracellular levels of TNFSF2 in human breast cancer. How or if estradiol may affect other members of the TNFSF/TNFRSF family is less studied. Estradiol has been shown to increase the expression of TNFSF2, TNF-R1, and TNF-R2 in pituitary cells and fibroblasts from breast tissue (55, 56). Our present data are in line with these previous findings as TNF and soluble TNF-R1 and soluble TNF-R2 correlated significantly with estradiol in normal breast tissue and exhibited increased levels in premenopausal breast tissue as compared to postmenopausal breasts. Regarding TNFSF14, previous data suggest that ovariectomy induces an increase of the protein in bone and circulating T-cells in women (57),. These data contrast with our findings where there was a highly significant positive correlation between local estradiol and TNFSF14 and over twice as high levels in premenopausal breasts compared to postmenopausal breast tissue. In bone tissue estradiol has been found to regulated TNFRSF11B at the transcriptional levels (58). However, our data do not support an estrogen regulation of TNFRSF11B in breast tissue as no differences were found between pre- and postmenopausal women an no correlations with estradiol was detected.
To elucidate whether our findings of correlations between estradiol and several of the TNFSFs/TNFRSFs were breast tissue specific we also measured these proteins in abdominal s.c. fat. Our data suggest that estradiol may affect levels of sTNFRSF6B, sTNFRSF4, TNFSF14, sTNFRSF10B, sTNFRSF10C, and sTNF-R2 systemically as these proteins correlated with estradiol both in breast tissue and abdominal s.c. fat. The levels of TNFSF2, sTNFRSF3, sTNFRSF9, TNFSF10, sTNF-R1, TNFSF13, sTNFRSF14, TNFSF13B, however, correlated with estradiol in breast tissue only suggesting that these factors may be under hormonal control in a tissue specific manner.
The relationship between mammographic density and the TNFSF/TNFRSF family of proteins is to a large extent unexplored and limited data are available. Immunohistochemical determination of TNFSF2 has failed to show any association with mammographic density (59), which is in line with our data not showing any association with breast density quantified by LTF and TNFSF2.
TNFSF/TNFRSF family of proteins may induce both pro-tumorigenic and tumor-suppressive effects in various tissues and organs. Further studies are warranted to fully understand these pathways in breast cancer and normal breast tissue at high risk of cancer. Our data clearly show that these proteins are dysregulated in both breast cancer and dense breast tissue of postmenopausal women and that several of the proteins are under estrogen control in breast tissue. Our data indeed suggest these pathways may be worthwhile to further elucidate as targets for therapy and prevention of breast cancer.
Data availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Regional Ethical Review Board of Linköping. The patients/participants provided their written informed consent to participate in this study. The animal study was reviewed and approved by The Institutional Animal Ethics Committee at Linköping University.
Author contributions
CD designed the project and performed all microdialysis investigations. AA carried out sample preparation and participated in the animal work. CD, JE, MZ, AA, PL, MF analyzed the data and prepared and finally approved the manuscript.
Funding
This work was supported by grants to C.D. from the Swedish Cancer Society (2018/464), the Swedish Research Council (2018-02584), LiU-Cancer, and ALF of Linköping University Hospital.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to thank Ann-Christine Andersson of the Department of Oncology, Linköping University Hospital for providing excellent technical assistance and Anna Rzepecka MD and the staff of the Mammography Department, Linköping University Hospital, for identifying subjects with different mammographic densities.
References
- 1. Boyd NF, Martin LJ, Bronskill M, Yaffe MJ, Duric N, Minkin S. Breast Tissue Composition and Susceptibility to Breast Cancer. J Natl Cancer Inst (2010) 102(16):1224–37. doi: 10.1093/jnci/djq239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pettersson A, Hankinson SE, Willett WC, Lagiou P, Trichopoulos D, Tamimi RM. Nondense Mammographic Area and Risk of Breast Cancer. Breast Cancer Res (2011) 13(5):R100. doi: 10.1186/bcr3041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boyd NF, Guo H, Martin LJ, Sun L, Stone J, Fishell E, et al. Mammographic Density and the Risk and Detection of Breast Cancer. N Engl J Med (2007) 356(3):227–36. doi: 10.1056/NEJMoa062790 [DOI] [PubMed] [Google Scholar]
- 4. Lin SJ, Cawson J, Hill P, Haviv I, Jenkins M, Hopper JL, et al. Image-Guided Sampling Reveals Increased Stroma and Lower Glandular Complexity in Mammographically Dense Breast Tissue. Breast Cancer Res Treat (2011) 128(2):505–16. doi: 10.1007/s10549-011-1346-0 [DOI] [PubMed] [Google Scholar]
- 5. Alowami S, Troup S, Al-Haddad S, Kirkpatrick I, Watson PH. Mammographic Density Is Related to Stroma and Stromal Proteoglycan Expression. Breast Cancer Res (2003) 5(5):R129–35. doi: 10.1186/bcr622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ghosh K, Brandt KR, Reynolds C, Scott CG, Pankratz VS, Riehle DL, et al. Tissue Composition of Mammographically Dense and Non-Dense Breast Tissue. Breast Cancer Res Treat (2012) 131(1):267–75. doi: 10.1007/s10549-011-1727-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hawes D, Downey S, Pearce CL, Bartow S, Wan P, Pike MC, et al. Dense Breast Stromal Tissue Shows Greatly Increased Concentration of Breast Epithelium But No Increase in its Proliferative Activity. Breast Cancer Res (2006) 8(2):R24. doi: 10.1186/bcr1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khan QJ, Kimler BF, O'Dea AP, Zalles CM, Sharma P, Fabian CJ. Mammographic Density Does Not Correlate With Ki-67 Expression or Cytomorphology in Benign Breast Cells Obtained by Random Periareolar Fine Needle Aspiration From Women at High Risk for Breast Cancer. Breast Cancer Res (2007) 9(3):R35. doi: 10.1186/bcr1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kaaks R, Rinaldi S, Key TJ, Berrino F, Peeters PH, Biessy C, et al. Postmenopausal Serum Androgens, Oestrogens and Breast Cancer Risk: The European Prospective Investigation Into Cancer and Nutrition. Endocr Relat Cancer (2005) 12(4):1071–82. doi: 10.1677/erc.1.01038 [DOI] [PubMed] [Google Scholar]
- 10. Beral V. Breast Cancer and Hormone-Replacement Therapy in the Million Women Study. Lancet (2003) 362(9382):419–27. doi: 10.1016/S0140-6736(03)14065-2 [DOI] [PubMed] [Google Scholar]
- 11. Wallach D. The Tumor Necrosis Factor Family: Family Conventions and Private Idiosyncrasies. Cold Spring Harb Perspect Biol (2018) 10(10):1–22. doi: 10.1101/cshperspect.a028431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liubomirski Y, Lerrer S, Meshel T, Rubinstein-Achiasaf L, Morein D, Wiemann S, et al. Tumor-Stroma-Inflammation Networks Promote Pro-Metastatic Chemokines and Aggressiveness Characteristics in Triple-Negative Breast Cancer. Front Immunol (2019) 10:757. doi: 10.3389/fimmu.2019.00757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Abrahamsson A, Rzepecka A, Romu T, Borga M, Leinhard OD, Lundberg P, et al. Dense Breast Tissue in Postmenopausal Women is Associated With a Pro-Inflammatory Microenvironment In Vivo. Oncoimmunology (2016) 5(10):e1229723. doi: 10.1080/2162402X.2016.1229723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sickles E, D’Orsi C, Bassett L. ACR BI-RADS® Mammography. In: ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System. Reston, VA: American College of Radiology; (2013). [Google Scholar]
- 15. Aberg UW, Saarinen N, Abrahamsson A, Nurmi T, Engblom S, Dabrosin C. Tamoxifen and Flaxseed Alter Angiogenesis Regulators in Normal Human Breast Tissue In Vivo. PloS One (2011) 6(9):e25720. doi: 10.1371/journal.pone.0025720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bendrik C, Dabrosin C. Estradiol Increases IL-8 Secretion of Normal Human Breast Tissue and Breast Cancer In Vivo. J Immunol (2009) 182(1):371–8. doi: 10.4049/jimmunol.182.1.371 [DOI] [PubMed] [Google Scholar]
- 17. Dabrosin C. Increase of Free Insulin-Like Growth Factor-1 in Normal Human Breast In Vivo Late in the Menstrual Cycle. Breast Cancer Res Treat (2003) 80(2):193–8. doi: 10.1023/A:1024575103524 [DOI] [PubMed] [Google Scholar]
- 18. Dabrosin C. Increased Extracellular Local Levels of Estradiol in Normal Breast In Vivo During the Luteal Phase of the Menstrual Cycle. J Endocrinol (2005) 187(1):103–8. doi: 10.1677/joe.1.06163 [DOI] [PubMed] [Google Scholar]
- 19. Dabrosin C. Microdialysis - An In Vivo Technique for Studies of Growth Factors in Breast Cancer. Front Biosci (2005) 10:1329–35. doi: 10.2741/1622 [DOI] [PubMed] [Google Scholar]
- 20. Dabrosin C. Positive Correlation Between Estradiol and Vascular Endothelial Growth Factor But Not Fibroblast Growth Factor-2 in Normal Human Breast Tissue In Vivo. Clin Cancer Res (2005) 11(22):8036–41. doi: 10.1158/1078-0432.CCR-05-0977 [DOI] [PubMed] [Google Scholar]
- 21. Dabrosin C. Sex Steroid Regulation of Angiogenesis in Breast Tissue. Angiogenesis (2005) 8(2):127–36. doi: 10.1007/s10456-005-9002-0 [DOI] [PubMed] [Google Scholar]
- 22. Garvin S, Dabrosin C. In Vivo Measurement of Tumor Estradiol and Vascular Endothelial Growth Factor in Breast Cancer Patients. BMC Cancer (2008) 8:73. doi: 10.1186/1471-2407-8-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nilsson UW, Abrahamsson A, Dabrosin C. Angiogenin Regulation by Estradiol in Breast Tissue: Tamoxifen Inhibits Angiogenin Nuclear Translocation and Antiangiogenin Therapy Reduces Breast Cancer Growth In Vivo. Clin Cancer Res (2010) 16(14):3659–69. doi: 10.1158/1078-0432.CCR-10-0501 [DOI] [PubMed] [Google Scholar]
- 24. Abrahamsson A, Dabrosin C. Tissue Specific Expression of Extracellular microRNA in Human Breast Cancers and Normal Human Breast Tissue In Vivo. Oncotarget (2015) 6(26):22959–69. doi: 10.18632/oncotarget.4038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dabrosin C, Hallstrom A, Ungerstedt U, Hammar M. Microdialysis of Human Breast Tissue During the Menstrual Cycle. Clin Sci (Lond) (1997) 92(5):493–6. doi: 10.1042/cs0920493 [DOI] [PubMed] [Google Scholar]
- 26. Dabrosin C, Margetts PJ, Gauldie J. Estradiol Increases Extracellular Levels of Vascular Endothelial Growth Factor In Vivo in Murine Mammary Cancer. Int J Cancer (2003) 107(4):535–40. doi: 10.1002/ijc.11398 [DOI] [PubMed] [Google Scholar]
- 27. Garvin S, Dabrosin C. Tamoxifen Inhibits Secretion of Vascular Endothelial Growth Factor in Breast Cancer In Vivo. Cancer Res (2003) 63(24):8742–8. [PubMed] [Google Scholar]
- 28. Lindahl G, Saarinen N, Abrahamsson A, Dabrosin C. Tamoxifen, Flaxseed, and the Lignan Enterolactone Increase Stroma- and Cancer Cell-Derived IL-1Ra and Decrease Tumor Angiogenesis in Estrogen-Dependent Breast Cancer. Cancer Res (2011) 71(1):51–60. doi: 10.1158/0008-5472.CAN-10-2289 [DOI] [PubMed] [Google Scholar]
- 29. Abrahamsson A, Rodriguez GV, Dabrosin C. Fulvestrant-Mediated Attenuation of the Innate Immune Response Decreases ER(+) Breast Cancer Growth In Vivo More Effectively Than Tamoxifen. Cancer Res (2020) 80(20):4487–99. doi: 10.1158/0008-5472.CAN-20-1705 [DOI] [PubMed] [Google Scholar]
- 30. Abrahamsson A, Rzepecka A, Dabrosin C. Equal Pro-Inflammatory Profiles of CCLs, CXCLs, and Matrix Metalloproteinases in the Extracellular Microenvironment In Vivo in Human Dense Breast Tissue and Breast Cancer. Front Immunol (2017) 8:1994. doi: 10.3389/fimmu.2017.01994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mijic S, Dabrosin C. Platelet Activation in Situ in Breasts at High Risk of Cancer: Relationship With Mammographic Density and Estradiol. J Clin Endocrinol Metab (2020) 106(2):485–500. doi: 10.1210/clinem/dgaa820 [DOI] [PubMed] [Google Scholar]
- 32. Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF Receptor Superfamilies: Integrating Mammalian Biology. Cell (2001) 104(4):487–501. doi: 10.1016/S0092-8674(01)00237-9 [DOI] [PubMed] [Google Scholar]
- 33. Dostert C, Grusdat M, Letellier E, Brenner D. The TNF Family of Ligands and Receptors: Communication Modules in the Immune System and Beyond. Physiol Rev (2019) 99(1):115–60. doi: 10.1152/physrev.00045.2017 [DOI] [PubMed] [Google Scholar]
- 34. Martinez-Reza I, Diaz L, Garcia-Becerra R. Preclinical and Clinical Aspects of TNF-Alpha and its Receptors TNFR1 and TNFR2 in Breast Cancer. J BioMed Sci (2017) 24(1):90. doi: 10.1186/s12929-017-0398-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lienard D, Ewalenko P, Delmotte JJ, Renard N, Lejeune FJ. High-Dose Recombinant Tumor Necrosis Factor Alpha in Combination With Interferon Gamma and Melphalan in Isolation Perfusion of the Limbs for Melanoma and Sarcoma. J Clin Oncol (1992) 10(1):52–60. doi: 10.1200/JCO.1992.10.1.52 [DOI] [PubMed] [Google Scholar]
- 36. Lin WW, Hsieh SL. Decoy Receptor 3: A Pleiotropic Immunomodulator and Biomarker for Inflammatory Diseases, Autoimmune Diseases and Cancer. Biochem Pharmacol (2011) 81(7):838–47. doi: 10.1016/j.bcp.2011.01.011 [DOI] [PubMed] [Google Scholar]
- 37. Yang CR, Hsieh SL, Teng CM, Ho FM, Su WL, Lin WW. Soluble Decoy Receptor 3 Induces Angiogenesis by Neutralization of TL1A, a Cytokine Belonging to Tumor Necrosis Factor Superfamily and Exhibiting Angiostatic Action. Cancer Res (2004) 64(3):1122–9. doi: 10.1158/0008-5472.CAN-03-0609 [DOI] [PubMed] [Google Scholar]
- 38. Jiang M, Lin X, He R, Lin X, Liang L, Tang R, et al. Decoy Receptor 3 (DcR3) as a Biomarker of Tumor Deterioration in Female Reproductive Cancers: A Meta-Analysis. Med Sci Monit (2016) 22:1850–7. doi: 10.12659/MSM.896226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cardoso Alves L, Corazza N, Micheau O, Krebs P. The Multifaceted Role of TRAIL Signaling in Cancer and Immunity. FEBS J (2021) 288(19):5530–54. doi: 10.1111/febs.15637 [DOI] [PubMed] [Google Scholar]
- 40. Vredevoogd DW, Kuilman T, Ligtenberg MA, Boshuizen J, Stecker KE, de Bruijn B, et al. Augmenting Immunotherapy Impact by Lowering Tumor TNF Cytotoxicity Threshold. Cell (2019) 178(3):585–99 e15. doi: 10.1016/j.cell.2019.06.014 [DOI] [PubMed] [Google Scholar]
- 41. Johansson-Percival A, He B, Li ZJ, Kjellen A, Russell K, Li J, et al. De Novo Induction of Intratumoral Lymphoid Structures and Vessel Normalization Enhances Immunotherapy in Resistant Tumors. Nat Immunol (2017) 18(11):1207–17. doi: 10.1038/ni.3836 [DOI] [PubMed] [Google Scholar]
- 42. He B, Johansson-Percival A, Backhouse J, Li J, Lee GYF, Hamzah J, et al. Remodeling of Metastatic Vasculature Reduces Lung Colonization and Sensitizes Overt Metastases to Immunotherapy. Cell Rep (2020) 30(3):714–24 e5. doi: 10.1016/j.celrep.2019.12.013 [DOI] [PubMed] [Google Scholar]
- 43. Yu G, Boone T, Delaney J, Hawkins N, Kelley M, Ramakrishnan M, et al. APRIL and TALL-I and Receptors BCMA and TACI: System for Regulating Humoral Immunity. Nat Immunol (2000) 1(3):252–6. doi: 10.1038/79802 [DOI] [PubMed] [Google Scholar]
- 44. Remouchamps C, Boutaffala L, Ganeff C, Dejardin E. Biology and Signal Transduction Pathways of the Lymphotoxin-Alphabeta/LTbetaR System. Cytokine Growth Factor Rev (2011) 22(5-6):301–10. doi: 10.1016/j.cytogfr.2011.11.007 [DOI] [PubMed] [Google Scholar]
- 45. Villa-Morales M, Fernandez-Piqueras J. Targeting the Fas/FasL Signaling Pathway in Cancer Therapy. Expert Opin Ther Targets (2012) 16(1):85–101. doi: 10.1517/14728222.2011.628937 [DOI] [PubMed] [Google Scholar]
- 46. Moran AE, Kovacsovics-Bankowski M, Weinberg AD. The TNFRs OX40, 4-1BB, and CD40 as Targets for Cancer Immunotherapy. Curr Opin Immunol (2013) 25(2):230–7. doi: 10.1016/j.coi.2013.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chaudhary PM, Eby M, Jasmin A, Bookwalter A, Murray J, Hood L. Death Receptor 5, a New Member of the TNFR Family, and DR4 Induce FADD-Dependent Apoptosis and Activate the NF-kappaB Pathway. Immunity (1997) 7(6):821–30. doi: 10.1016/S1074-7613(00)80400-8 [DOI] [PubMed] [Google Scholar]
- 48. Steinberg MW, Cheung TC, Ware CF. The Signaling Networks of the Herpesvirus Entry Mediator (TNFRSF14) in Immune Regulation. Immunol Rev (2011) 244(1):169–87. doi: 10.1111/j.1600-065X.2011.01064.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schon S, Flierman I, Ofner A, Stahringer A, Holdt LM, Kolligs FT, et al. Beta-Catenin Regulates NF-kappaB Activity via TNFRSF19 in Colorectal Cancer Cells. Int J Cancer (2014) 135(8):1800–11. doi: 10.1002/ijc.28839 [DOI] [PubMed] [Google Scholar]
- 50. Spanjaard RA, Whren KM, Graves C, Bhawan J. Tumor Necrosis Factor Receptor Superfamily Member TROY is a Novel Melanoma Biomarker and Potential Therapeutic Target. Int J Cancer (2007) 120(6):1304–10. doi: 10.1002/ijc.22367 [DOI] [PubMed] [Google Scholar]
- 51. Riggs BL. The Mechanisms of Estrogen Regulation of Bone Resorption. J Clin Invest (2000) 106(10):1203–4. doi: 10.1172/JCI11468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mackern-Oberti JP, Jara EL, Riedel CA, Kalergis AM. Hormonal Modulation of Dendritic Cells Differentiation, Maturation and Function: Implications for the Initiation and Progress of Systemic Autoimmunity. Arch Immunol Ther Exp (Warsz) (2017) 65(2):123–36. doi: 10.1007/s00005-016-0418-6 [DOI] [PubMed] [Google Scholar]
- 53. Cutolo M, Sulli A, Straub RH. Estrogen Metabolism and Autoimmunity. Autoimmun Rev (2012) 11(6-7):A460–4. doi: 10.1016/j.autrev.2011.11.014 [DOI] [PubMed] [Google Scholar]
- 54. Jamdade VS, Mundhe NA, Kumar P, Tadla V, Lahkar M. Raloxifene Inhibits NF-kB Pathway and Potentiates Anti-Tumour Activity of Cisplatin With Simultaneous Reduction in Its Nephrotoxictiy. Pathol Oncol Res (2016) 22(1):145–53. doi: 10.1007/s12253-015-9988-6 [DOI] [PubMed] [Google Scholar]
- 55. Deb S, Amin S, Imir AG, Yilmaz MB, Suzuki T, Sasano H, et al. Estrogen Regulates Expression of Tumor Necrosis Factor Receptors in Breast Adipose Fibroblasts. J Clin Endocrinol Metab (2004) 89(8):4018–24. doi: 10.1210/jc.2004-0127 [DOI] [PubMed] [Google Scholar]
- 56. Zaldivar V, Magri ML, Zarate S, Jaita G, Eijo G, Radl D, et al. Estradiol Increases the Expression of TNF-Alpha and TNF Receptor 1 in Lactotropes. Neuroendocrinology (2011) 93(2):106–13. doi: 10.1159/000323760 [DOI] [PubMed] [Google Scholar]
- 57. Brunetti G, Storlino G, Oranger A, Colaianni G, Faienza MF, Ingravallo G, et al. LIGHT/TNFSF14 Regulates Estrogen Deficiency-Induced Bone Loss. J Pathol (2020) 250(4):440–51. doi: 10.1002/path.5385 [DOI] [PubMed] [Google Scholar]
- 58. Bord S, Ireland DC, Beavan SR, Compston JE. The Effects of Estrogen on Osteoprotegerin, RANKL, and Estrogen Receptor Expression in Human Osteoblasts. Bone (2003) 32(2):136–41. doi: 10.1016/S8756-3282(02)00953-5 [DOI] [PubMed] [Google Scholar]
- 59. Maskarinec G, Ju D, Fong J, Horio D, Chan O, Loo LWM, et al. Mammographic Density and Breast Tissue Expression of Inflammatory Markers, Growth Factors, and Vimentin. BMC Cancer (2018) 18(1):1191. doi: 10.1186/s12885-018-5088-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.