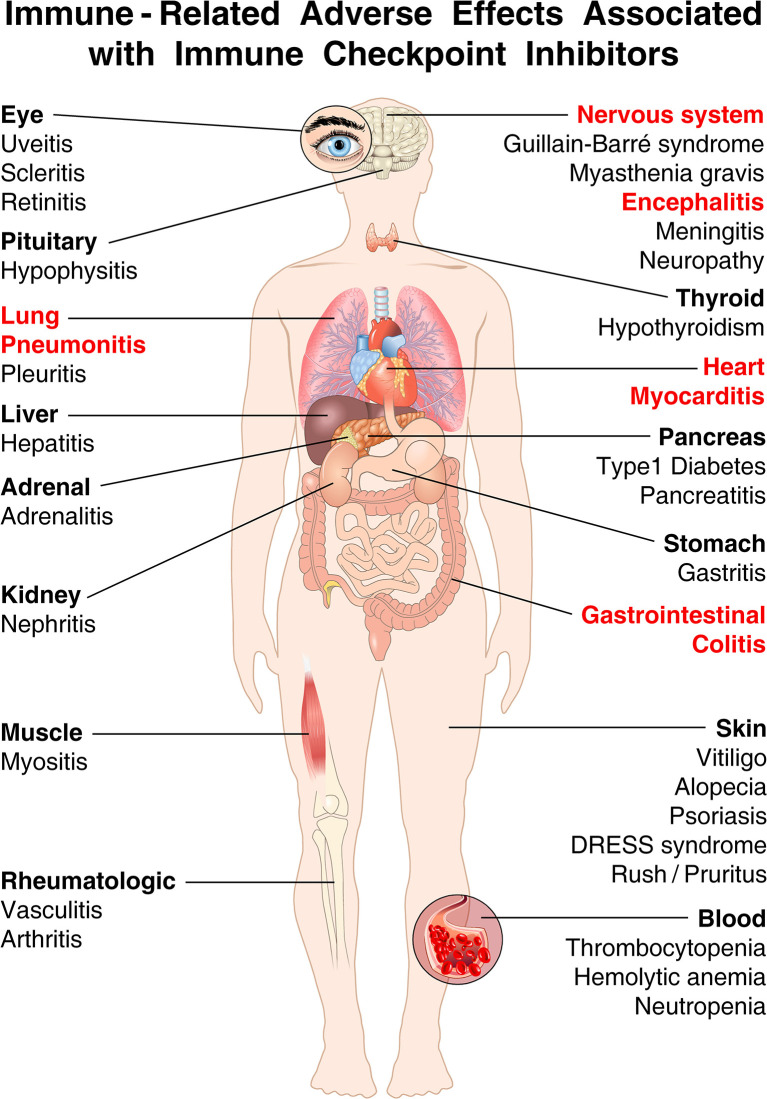
Figure 3.
The spectrum of organs affected by irAEs associated with immune checkpoint inhibitors (ICIs) is very broad. Shown are the most common immune-related adverse events (irAEs) that clinicians can encounter in cancer patients treated with ICIs. irAEs contributing to most fatalities are highlighted in red [modified from (19)]. ICI-associated diabetes is almost exclusively seen in patients treated with anti-PD-1/PD-L1 antibodies, and rarely with ipilimumab monotherapy (42, 118). By contrast, hypophysitis occurs more often in patients receiving ipilimumab (119). Colitis occurs more commonly with ipilimumab and with combined immune checkpoint blockade than with anti-PD-1/PD-L1 alone (112, 120). Endocrinopathies (such as hypothyroidism, hypophysitis, and adrenal insufficiency) and rheumatological disorders have the highest incidence of development into subacute/chronic toxicity (49). Endocrine toxicities, unlike many other irAEs, are not managed using high-dose glucocorticoids, which have no effect on either initial severity and resolution (121, 122). Although acute myocarditis was the first cardiovascular irAEs associated with ICIs (123), an important unanswered question relates to the long-term cardiovascular sequelae of ICIs (49, 56, 57).