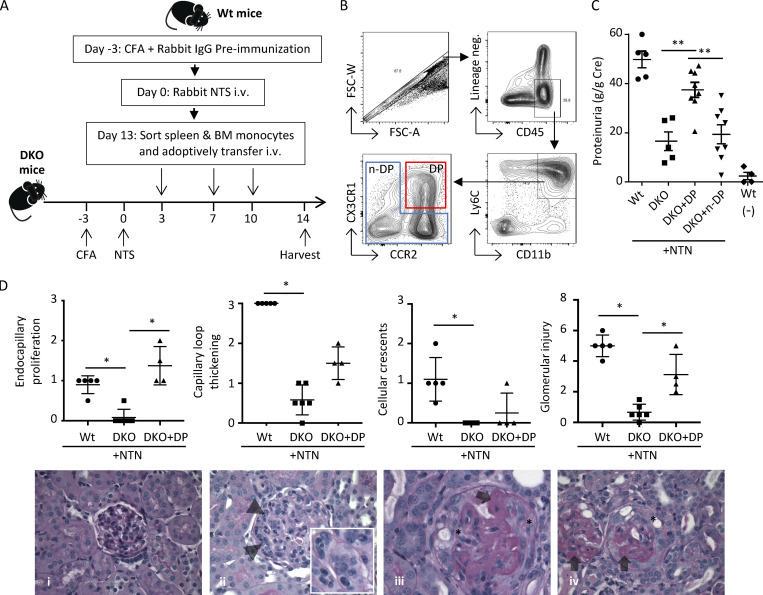
Figure 3.
Adoptively transferred Ly6C+CCR2+CX3CR1+ monocytes promote glomerular injury. (A) Schematic for adoptive transfer of cells and induction of NTN. Spleen/bone marrow monocytes were sorted from cohorts of Wt donor mice on day 13 after induction of NTN and injected at indicated days into Ccr2 and Cx3cr1 DKO recipient mice that had been immunized with CFA and NTS to induce NTN. Urine samples and both kidneys of DKO recipient mice were harvested on day 14 for analysis of proteinuria and histological scoring of GN. (B) Flow cytometry gating strategy for sorting CD45+, lineage-negative (CD3−NK−Ly6G−CD11c−), CD11b+Ly6C+CX3CR1+CCR2+ DP (red box), and the remaining non-DP fractions (n-DP, blue box). FSC, forward scatter. (C) Proteinuria in Wt mice with (n = 5) and without (n = 4) NTN, DKO mice (n = 5), and recipient DKO mice transferred with DP (n = 8) or n-DP (n = 8) cells. Two independent experiments were performed. (D) Histological scores for endocapillary proliferation, capillary loop thickening, cellular crescents, and glomerular injury in Wt, DKO, and DKO + DP cells on day 14 after NTN induction. Representative histological images of periodic acid–Schiff-stained slides are shown. (i) A normal glomerulus with normal glomerular capillary loops (thickness) and normocellular without evidence of an active glomerulitis (i.e., endocapillary proliferation or cellular crescents). (ii) A glomerulus with global endocapillary proliferation, with inflammatory cells within the glomerular capillary loops indicated by arrowheads. (iii and iv) Glomeruli with severe glomerular capillary loop thickening (indicated with arrows) and extracapillary proliferation of cellular crescents (indicated with asterisks). Original magnifications for i, ii, and iv, 600×; for iii, 400×. Data are mean ± SEM. *, P < 0.05; **, P < 0.01.