Abstract
Background:
Sodium-glucose cotransporter 2 inhibitors (SGLT2i) are a group of antidiabetic medications with a favourable cardiovascular, renal and overall safety profile. Given the limited treatment options available for neurological disorders, it is important to determine whether the pleiotropic effects of SGLT2i can be utilised in their prevention and management.
Methods:
All articles published before 20 March 2021 were systematically searched in MEDLINE, EMBASE, Scopus, Web of Science, APA PsycINFO and ClinicalTrials.gov. Overall, 1395 titles were screened, ultimately resulting in 160 articles being included in the qualitative analysis. Screening and data extraction were conducted by two independent authors and studies were excluded if they were not an original research study.
Findings:
Of the 160 studies, 134 addressed stroke, 19 cognitive impairment, 4 epilepsy and 4 movement disorders, encompassing a range from systematic reviews and randomised controlled trials to bioinformatic and animal studies. Most animal studies demonstrated significant improvements in behavioural and neurological deficits, which were reflected in beneficial changes in neurovascular units, synaptogenesis, neurotransmitter levels and target receptors’ docking energies. The evidence from the minority clinical literature was conflicting and many studies did not reach statistical significance.
Interpretation:
SGLT2i may exert neurological benefits through three mechanisms: reduction in cardiovascular risk factors, augmentation of ketogenesis and anti-inflammatory pathways. Most clinical studies were observational, meaning that a causal relationship could not be established, while randomised controlled trials were heterogeneous and powered to detect cardiovascular or renal outcomes. We suggest that a longitudinal study should be conducted and specifically powered to detect neurological outcomes.
Keywords: cognitive impairment, epilepsy, Parkinson’s disease, scoping review, sodium-glucose cotransporter 2 inhibitors (SGLT2i), stroke
Introduction
Sodium-glucose cotransporter 2 inhibitors (SGLT2i) are a class of antidiabetic medications, including canagliflozin, dapagliflozin and empagliflozin, that act by blocking the re-uptake of glucose in the proximal renal tubule, thus inducing glycosuria and improving glycaemic control. Their beneficial impact on cardiovascular events such as stroke and heart failure, and their benefits in reducing the progression of chronic kidney disease are well reported in the literature. 1 However, there is limited literature describing the impact of SGLT2i on prevalent neurological disorders such as epilepsy, dementia and Parkinson’s disease (PD).
This is an important relationship to explore for several reasons. Neurological disorders often lead to a significantly worse quality of life, for example, stroke causes lifelong physical, functional and cognitive disability. 2 Moreover, dementia was recently found to be the fifth leading cause of deaths globally. Its prevalence is increasing at an alarming rate due to an ageing population, which will result in profound medical, social and economic consequences if preventive measures are not enacted. 3 Unfortunately, we have still not found a treatment for dementia that is strongly disease-modifying on its own, although a combination of treatments has been found to have a significant effect. 4 Therefore, it is pivotal to investigate whether we can utilise the pleiotropic effects of other medications available on the market, for the prevention and management of neurological disorders.
Both animal and clinical studies have established a link between type 2 diabetes mellitus (T2DM) and neurological disorders, with T2DM being a major risk factor for stroke and Alzheimer’s disease (AD). 5 Intriguingly, pancreatic islet amyloid polypeptide has been found to co-localise with amyloid-beta plaques in the brain, and the former promotes the oligomerisation of the latter. 5 This suggests one potential mechanism linking T2DM and AD, with the corollary that targeting T2DM might mitigate the neurodegeneration seen in AD.
SGLT2 receptors are a viable target for the management of neurological disorders, given their abundance throughout the central nervous system, and influence on neuron membrane potential through the modulation of sodium transport. 6 Moreover, these receptors are specifically expressed in the blood–brain barrier (BBB) and activated by post-ischaemic cerebral hyperglycaemia, which in turn exacerbates neuronal damage. 7 In an animal model of stroke, the SGLT2 inhibitor phlorizin can reverse this central hyperglycaemia and reduce cerebral oedema. 8 The ability of SGLT2i to cross the BBB, due to their lipid solubility, further supports the therapeutic potential of SGLT2i for neurological disorders.
The glycosuria and reduction in plasma glucose induced by SGLT2i leads to a shift in substrate utilisation from carbohydrates to fatty acid oxidation, consequently leading to ketogenesis. This may have neurological benefits, which is reflected in the efficacy of a ketogenic diet in the management of refractory epilepsy, 9 the improvement of cognitive function in AD patients taking a ketogenic medication 10 and the significant improvement of motor and non-motor symptoms in PD observed with a ketogenic diet. 11 The neuroprotective effects of ketogenesis are thought to be due to its ability to bypass defects in mitochondrial respiratory complexes and overall reduce oxidative damage in neurons. 12 This lends further support for exploring the neurological benefits of SGLT2i, possibly through the augmentation of ketogenesis.
Given these premises, the aim of this scoping review is to evaluate and summarise all available literature on the impact of SGLT2i on neurological disorders.
Methods
This review was conducted and reported in accordance with the PRISMA extension for scoping reviews (a completed checklist can be found in Supplemental Appendix 1). 13 Comprehensive literature searches of MEDLINE, EMBASE, Scopus, Web of Science, APA PsycINFO and ClinicalTrials.gov were performed to include studies from inception to 20 March 2021. A MeSH search and free-text search were carried out using keywords relating to SGLT2i and neurological disorders. The commonly used SGLT2i are shown in Table 1. The full search strategy can be found in Supplemental Appendix 2.
Table 1.
FDA- and EMA-approved sodium-glucose cotransporter 2 inhibitors (SGLT2i).
| Generic name | Canagliflozin | Dapagliflozin | Empagliflozin |
|---|---|---|---|
| Brand name | Invokana | Farxiga | Jardiance |
| Available doses (mg) | 100, 300 | 5, 10 | 10, 25 |
| Administration | Once a day before first meal | Once a day | Once a day |
| SGLT2 to SGLT1 selectivity | 250:1 | 1200:1 | 2500:1 |
| AChE inhibition Ki (µM) | 0.13 | 25.02 | 0.18 |
AChE, acetylcholinesterase; EMA, European Medicines Agency; FDA, The US Food and Drug Administration; Ki, inhibitor constant.
Additional studies were identified by scanning the references of included studies and other narrative reviews. The inclusion criteria were as follows: (a) any research study, such as systematic reviews, meta-analyses, randomised controlled trials, case series, case reports, case–control studies, cohort studies, animal studies, in vitro studies, simulation/docking studies; (b) any research study that explored the impact of SGLT2i on any neurological disorder or their respective biochemical targets. Studies were excluded if (a) the data specifically relating to the neurological disorder could not be reliably extracted from a pooled dataset, or (b) not an original research article, such as narrative reviews and letters to the editor. No limitation filters were applied to the study type, language or publication date, to include a range of different studies.
Two authors (T.T. and J.S.Y.H.) independently screened and assessed all titles, abstracts and full texts. Corresponding authors were contacted for full texts or missing data. Full texts of articles meeting the inclusion criteria were examined and relevant studies were analysed for data extraction. Any disagreements between the two authors were resolved through discussion and consensus reached.
Data extraction was undertaken independently by two researchers (T.T. and J.S.Y.H.) using a data extraction template, which can be found in Supplemental Appendix 3. The results of each study were summarised into one sentence, including important statistics that were reported. Studies were then categorised and analysed by neurological disorder.
Ethical approval by the institutional review board was not required as only published data were included and there was no funding source for this study.
Results
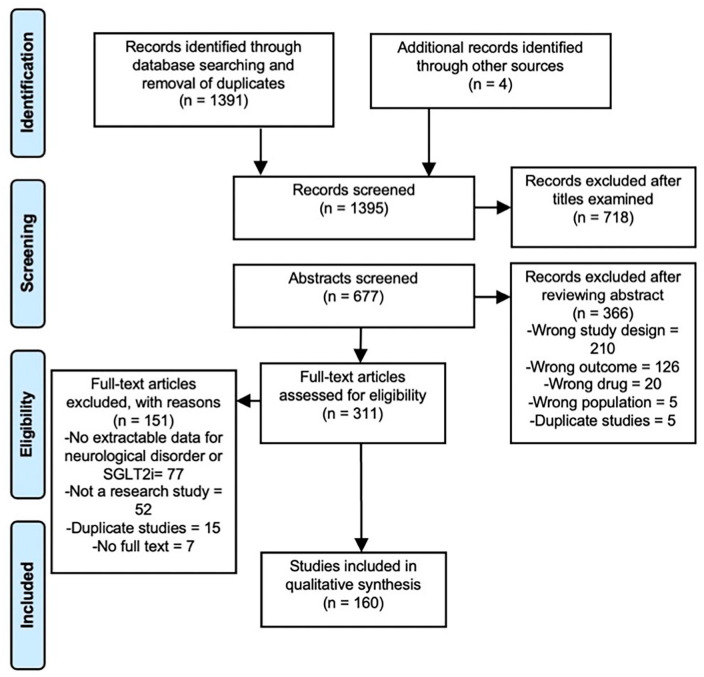
The primary search resulted in 1391 potentially relevant studies following the removal of duplicates. Four additional studies were identified through review of reference lists and other narrative reviews. Ultimately, 160 studies were included in this scoping review (Figure 1). These studies were categorised according to the neurological disorder: stroke, cognitive impairment, epilepsy and movement disorders.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram for search strategy.
Cognitive impairment
Our systematic search identified 19 studies exploring the impact of SGLT2i on cognitive impairment, and 11 studies were performed in animal models of diabetic cognitive impairment and AD (full references in the Supplementary Material ). Streptozotocin or a high-fat diet was used to induce diabetes mellitus in rodents, as the impaired insulin signalling leads to a combination of defects in autonomic function and neuroinflammatory pathways, which ultimately results in impaired cognition. 14 Mutations in the amyloid precursor protein and presenilin-1 genes were used to create transgenic animal models of AD.
In all 11 studies, the rodent models predictably demonstrated diminished performance on memory tests such as the Morris Water Maze and novel object recognition test. Different SGLT2i were studied: canagliflozin, 15 luseogliflozin, 16 dapagliflozin, 17 empagliflozin18,19 and phlorizin, a non-selective SGLT1/2 inhibitor. 20 All SGLT2i-treated disease models showed significant improvement in memory tests.
Three studies focused on the mechanisms underlying the relationship between SGLT2I and cognition. First, Wang et al. demonstrated a 50% reduction in reactive oxygen species (ROS) in diabetic rats treated with luseogliflozin over a 4-month period. The proposed explanation for this reduction was an improvement in mitochondrial function. 16 Second, SGLT2i-treated models demonstrated a histological improvement in the neurovascular restructuring that is normally observed in cognitive decline. Specifically, SGLT2i reduced leakage of the BBB, reduced microglia burden and prevented myelin remodelling. 18 In parallel, increased brain-derived neurotrophic factor and synaptogenesis were observed in SGLT2i-treated models. 17 These changes were macroscopically manifested by reduced cortical thinning, haemorrhages and senile plaques. 19 Third, SGLT2i-treated mice showed significantly lower acetylcholinesterase (AChE) levels compared with placebo-treated mice. This increased the availability of acetylcholine and led to improvement in cognitive tasks. 15 The systematic review conducted by Panchal et al. 21 summarised these findings while highlighting that SGLT2i can increase monoamine levels to an extent comparable to galantamine therapy via reduction in AChE levels.
Among the 19 studies, 5 were bioinformatic studies investigating the docking of SGLT2i with molecules involved in the pathophysiology of AD, such as AChE. For example, Shaikh et al. 22 found that the docking energy between dapagliflozin and SGLT2, and between dapagliflozin and the CAS domain of AChE, were similar, which suggested that dapagliflozin may be a dual inhibitor of SGLT2 and AChE. Similarly, canagliflozin was found to have significant interactions with the S203 and H447 amino acid moieties of AChE. 23 Overall, all SGLT2 inhibitors formed stable complexes with AChE in these five docking studies. Alafnan 24 expanded upon these findings by revealing strong bonds between SGLT2i and other molecular targets implicated in the amyloidogenic and phosphorylation pathways of AD.
Of the 19 studies, two involved human participants.25,26 Perna et al. presented a randomised controlled trial of 39 elderly subjects with metformin-controlled T2DM and a mean age of 77. The addition of SGLT2i was compared with incretin regarding cognitive function over a 12-month period. There were no significant changes in cognition in either group, as measured by tests of working memory and attention. However, this study was limited by its small sample size, and the duration of follow-up might have been too short to detect any change in cognition. 26
Conversely, the cohort study by Wium-Andersen et al. 25 found that the use of SGLT2i was significantly associated with a lower risk of dementia in 11,619 patients with T2DM [odds ratio (OR), 0.58; 95% confidence interval (CI): 0.42–0.81]. While this was consistent with the animal studies suggesting a beneficial impact of SGLT2i on cognitive impairment, it is important to note that this is a retrospective observational study, which limits our ability to draw inferences about causation. 25
In contrast to all the previous studies, one of the 19 studies suggests that SGLT2 inhibition could have unfavourable effects on cognition. This study compared mice with and without a mutation in the SGLT2 gene and found that the mutation results in downregulation of amyloid-beta precursor-like protein, which is an important protein in synaptogenesis and central insulin homeostasis. The downregulation of this protein then led to memory impairment. 27
Epilepsy
Four studies addressed epilepsy,6,28–30 of which three were performed in animals,6,29,30 while the remaining human study was a case report. 28 In a study on 48 rats, Erdogan et al. 6 used pentylenetetrazol (PTZ) to induce seizures. Compared with vehicle and control arms, the administration of dapagliflozin significantly lowered spike wave percentage on intracranial electroencephalogram (EEG) monitoring. In addition, the severity of seizures was significantly reduced and the time to first myoclonic jerk was significantly shortened. The postulated explanations for better seizure control with this SGLT2i were that the inhibition of sodium and glucose into neurons results in reduced neuronal depolarisation and reduced glucose utilisation in the brain, therefore increasing seizure threshold due to reduced neuron excitability and limited metabolic resources for cellular respiration. 6
The case report by Blunck et al. 28 described a 42-year-old lady with super-refractory status epilepticus, which required propofol and midazolam infusions. The ketogenic diet alone failed to produce sustained ketosis for 2 weeks, prompting the initiation of dapagliflozin. Within 1 week of SGLT2i initiation, the patient entered a consistent state of ketosis for the first time in 65 days, enabling them to be weaned off the propofol infusion. However, it was difficult to determine whether the observed result was due to the sole effect of SGLT2i or its addition enabling the ketogenic diet to take effect. 28 Nevertheless, the case provided a support that SGLT2i can augment ketogenesis, which has been demonstrated to help in the treatment of refractory status epilepticus in previous studies. 9
A contrary argument for the use of SGLT2i for epilepsy was presented by Melo et al. in two similar animal studies that investigated the effect of phlorizin on pilocarpine-induced status epilepticus in male Wistar rats.29,30 In these studies, SGLT2i led to an increased severity of limbic seizures, quoting a significant 90% of animals in the phlorizin group having severe Racine’s scales scores, compared with 70% in vehicle. Furthermore, phlorizin significantly increased Fluoro-Jade C–positive cells in the hippocampus 24-h after status epilepticus, which was indicative of an extensive neurodegenerative process. The investigators argued that SGLT2 inhibition compromised neuronal survival due to less glucose entry into the cells with reduced adenosine triphosphate (ATP) generation. The compromised ATP production was believed to trigger cell death molecular cascades resulting in neurodegeneration.29,30 The conflicting results of Erdogan et al. and Melo et al. could be explained by the differences in the effects of phlorizin and dapagliflozin, since phlorizin inhibits SGLT2 as well as SGLT1, whereas dapagliflozin inhibits only SGLT2. 6
Movement disorders
Three animal studies and one docking study explored the impact of SGLT2i on diseases such as PD and Huntington’s Disease (HD). Anandhan et al. 31 found that the administration of phlorizin reduced the toxicity of paraquat in rat dopaminergic mesencephalic cell line N27 and marginally reduced cell death progression. This protective effect occurred through the joint activation of AMPK and reduced activation of the pentose phosphate pathway, leading to attenuated ROS production. 31 Expanding this to animal studies, Arab et al. 32 demonstrated that dapagliflozin attenuated neuronal injury and motor dysfunction in rotenone-induced rat model of PD, without inducing hypoglycaemia. In this study, dapagliflozin alleviated ROS production, boosted glial cell line–derived neurotrophic factor and suppressed cytokines, overall preserving the dopaminergic neurons and reducing accumulation of alpha synuclein. 32
Another emerging target for the management of PD is the A2A adenosine receptor (A2AAR), which commonly populates the basal ganglia. 33 Their antagonists have been proven to decrease off-time and troublesome dyskinesia while offering neuroprotection. 34 Ayoub et al. 35 examined this target in their docking study, revealing a stable complex formation between A2AAR and SGLT2i. Therefore, SGLT2i may be an effective antagonist against the A2AAR, although it may be intriguing to compare its docking energy to known antagonists such as istradefylline. 36
Only one study looked at the potential of SGLT2i in HD. 37 El-Sahar et al. recreated a rat model of HD by using a high single dose of 3-nitropropionic acid (3-NP) to cause acute striatal injury. This injury led to impairment in memory and locomotor functions, accompanied by reductions in acetylcholine and GABA, along with elevations in glutamate, aspartate and AChE activity. However, pre-treatment of 3-NP rats with dapagliflozin favourably reversed the behavioural and neurotransmitter levels significantly, providing evidence that dapagliflozin may have an inhibitive role against AChE. While 3-NP induced apoptosis, glycolysis and inflammation, it also induced an anti-autophagy response. However, pre-treatment with dapagliflozin induced autophagy through beclin-1 and LC3 expression, thus contributing to the striatal cell survival and subsequent observed behavioural improvement. 37
Stroke
A total of 134 articles reported stroke outcomes, which included 11 animal studies, three case reports, 58 cohort studies, 18 randomised controlled trials and 44 systematic reviews with meta-analysis (full references in the Supplementary Material). The randomised controlled trials, CANVAS Program, DECLARE-TIMI 58, CREDENCE, EMPA-REG OUTCOME, VERTIS CV and UTOPIA, compared the effect of SGLT2i versus placebo on cardiovascular outcomes in patients with T2DM, which are summarised in Table 2. Canagliflozin, dapagliflozin and empagliflozin had no significant effect on fatal or non-fatal stroke and ischaemic stroke. 38 The VERTIS CV trial compared ertugliflozin with placebo in 8246 patients and confirmed that ertugliflozin was not significantly associated with fatal or non-fatal stroke [hazard ratio (HR), 1.06; 95% CI: 0.82–1.37]. 39 In the UTOPIA study, there were no significant differences in stroke events or progression of carotid intima-media thickness between those on tofogliflozin and those on other non-SGLT2i antidiabetic medications. 40
Table 2.
Summary of the effect of SLT2i on stroke compared with other glucose-lowering drugs.
| All SGLT2i | Canagliflozin | Dapagliflozin | Empagliflozin | |
|---|---|---|---|---|
| All glucose-lowering drugs | 13 studies, >2.9 million patients: – 6 found a lower risk of stroke with SGLT2i – 6 found a higher risk of stroke with SGLT2i – 1 pharmacovigilance study showed higher reporting of all ischaemic stroke |
– | 1 study (n = 28,408), no significant differences in risk of stroke | – |
| Metformin | 1 study (n = 41,020) showed higher risk of ischaemic stroke (HR: 1.21) | – | – | – |
| Sulfonylurea | 1 study (n = 335,542) showed lower risk of stroke (HR: 0.73) | 1 study (n = 224,999), no significant difference in stroke outcomes | – | – |
| DPP4i | 7 studies (n > 700,000): 5 found a lower risk of stroke (HR: 0.46–0.86) 2 found no significant effect on stroke |
1 study (n = 224,999), no significant difference in stroke outcomes | 3 studies (n = 194,422) – 1 study (n = 128,066) showed reduced risk of stroke (HR: 0.82, 95% CI: 0.75–0.89) – 2 found no significant effect on stroke |
1 study (n = 39,169) showed no significant effect on ischaemic or haemorrhagic stroke |
| GLP-1RA | 3 studies (n > 177,000) No significant effect on ischaemic or haemorrhagic stroke |
1 study (n = 224,999), no significant difference in stroke outcomes | – | 1 study (n = 17,600) showed higher risk of ischaemic or haemorrhagic stroke (HR: 1.45) |
| Insulin | 1 study (n = 8984), insulin was associated with higher risk of stroke (HR: 7.21) | |||
| Placebo | – | CANVAS programme (n = 10,142) No significant effect on fatal or non-fatal stroke (HR: 0.87, 95% CI: 0.69–1.09) No significant effect on ischaemic stroke Reduction in haemorrhagic stroke (HR: 0.43, 95% CI: 0.20–0.89) CREDANCE trial (n = 4401): No significant effects on overall stroke, ischaemic stroke, haemorrhagic stroke or non-fatal stroke |
DECLARE-TIMI 58 (n = 17,160) No significant effect on ischaemic stroke (HR: 1.01, 95% CI: 0.84–1.21). Reduction in risk of ischaemic stroke in a subgroup of patients with DM for > 20 years (HR: 0.61, 95% CI: 0.38–1.00). |
EMPA-REG OUTCOME (n = 7020) No significant effects on overall stroke, fatal or non-fatal stroke, TIA On 5-year follow-up, higher risk of non-fatal stroke compared with liraglutide |
CI, confidence interval; DM, diabetes mellitus; DPP4i, dipeptidyl peptidadase-4 inhibitors; GLP-1RA, glucagon-like peptide 1 receptor agonists; HR, hazard ratio; n, number of participants in the study; SGLT2i, sodium-glucose cotransporter 2 inhibitors; TIA, transient ischaemic attack.
Of the 58 observational cohort studies, seven included patients on canagliflozin, 15 on dapagliflozin, 11 on empagliflozin and one each on tofogliflozin and ipragliflozin, while 23 did not specify the type of SGLT2i. Results from these studies are summarised in Table 2. In general, the effect of SGLT2i on stroke, compared with other glucose-lowering medications, has been mixed. One study showed higher risk of ischaemic stroke with SGLT2i than metformin, 41 but lower risk of stroke compared with sulfonylurea. 42 Comparing SGLT2i to dipeptidyl peptidadase-4 inhibitors (DPP4i), five studies showed a significant lower risk with SGLT2i (HR: 0.46–0.86). 43 Compared with glucagon-like peptide 1 receptor agonists (GLP-1RA), three studies found no significant differences in ischaemic or haemorrhagic stroke.44–46 Insulin use was associated with significantly higher risk of stroke compared with SGLT2i. 47 Comparing SGLT2i to all other glucose-lowering drugs, a similar number of studies found significant lower risks of stroke or no significant differences with SGLT2i.47–49
When canagliflozin was compared with non-SGLT2i (DPP4i, GLP-1RA and sulfonylurea), no significant difference in stroke outcome was observed. 44 On 1-year follow-up in adults aged ⩾65 years, 0.22% of patients on canagliflozin developed cerebral infarction, 0.07% had transient ischaemic attack (TIA) and 0.07% showed cerebrovascular stenosis. 50 Compared with other antidiabetic medications, dapagliflozin had a similar risk of stroke in patients with T2DM and cardiovascular disease (CVD) or at high risk of CVD. 49 Dapagliflozin had a reduced risk of stroke compared with DPP4i in one study, 51 while a similar but statistically insignificant trend were reported by two other studies.49,52 There were no significant differences in the risk of stroke between dapagliflozin and empagliflozin in a study of 12,681 patients with T2DM who were newly started on SGLT2i (HR: 1.15, 95% CI: 0.80–1.65). 53
There were 44 previous systematic reviews and meta-analyses comparing SGLT2i with placebo or other glucose-lowering medications for stroke outcomes. Of the 37 systematic reviews that compared SGLT2i with placebo, 33 found no significant differences in risk of stroke. While three reviews showed a lower risk of stroke with SGLT2i,54–56 one meta-analysis of 16 studies reported a higher risk of stroke associated with SGLT2i. 57 Similarly, nine meta-analyses of SGLT2i versus DPP4 inhibitors, GLP-1RA, metformin and standard care showed no significant differences. One meta-analysis of 14 studies found a lower risk of stroke in those taking SGLT2i compared with other glucose-lowering medications. 58
SGLT2i may have potential neuroprotective effects during cerebral ischaemia. Twelve in vivo and in vitro studies investigated the underlying mechanism of the effect on SGLT2i on stroke. Treatment with empagliflozin after bilateral carotid artery occlusion (BCAO)-induced ischaemia and reperfusion in rats reduced the infarct size and enhanced neurobehavioral functions in a dose-dependent manner. 59 EGT1442, a selective SGLT2i, dose-dependently reduced HbA1c and blood glucose concentration, and prolonged survival of the stroke-prone spontaneously hypertensive rats. 60 Intraperitoneal administration of phlorizin suppressed post-ischaemic hyperglycaemia, and intracerebroventricular or intraperitoneal administration of phlorizin significantly reduced pyknotic neuronal cell death and BCAO-induced spasticity on gait analysis. 61 When the function of SGLT in ischaemia induced by middle cerebral artery occlusion (MCAO) was evaluated in mice, Vemula et al. 62 found a time-dependent increase in [ 14 C]-methyl d-glucopyranoside (AMG), a specific, non-metabolised substrate of SGLT in mice exposed to focal ischaemia, which was reduced by phlorizin in a dose-dependent manner. This was similar to the observation of suppressed exacerbation by intracerebroventricular injection of glucose with the use of phlorizin. 8
The neuroprotective effects of SGLT2i are postulated to be due to its role in post-ischaemic hyperglycaemia, enhancement of hypoxia-inducible factor-1 (HIF-1α) and vascular endothelial growth factor (VEGF) pathway, as well as its effect on Aβ aggregation. Post-ischaemic hyperglycaemia develops 6 h after MCAO, and concomitant hydrogen peroxide and glucose injection worsened hydrogen peroxide–induced cell death. 63 Phlorizin increased neuronal survival 6 h after MCAO, but not immediately after MCAO, and suppressed cell death induced by hydrogen peroxide/glucose but not hydrogen peroxide alone. This suggests that SGLT may induce cell death through post-ischaemic hyperglycaemia, blocked by SGLT inhibitors. Sodium influx through cerebral SGLT also led to concentration-dependent decline in neuronal survival, ameliorated by phlorizin. 64 Empagliflozin is known to reduce neuronal caspase-3 expression, involved in apoptosis, in addition to upregulating HIF-1αα and VEGF post-ischaemic/reperfusion, and enhance recovery through angiogenesis and other neurotrophic effects. 59 Furthermore, pre-treatment with dapagliflozin resulted in decreased Aβ aggregation and BBB breakdown in male Wistar rats with cardiac ischaemic/reperfusion injury. 65
Discussion
Taken together, our scoping review demonstrates the wide array of potentially beneficial pleiotropic effects of SGLT2i on cognitive disorders, epilepsy, movement disorders and stroke. These effects were reflected in behavioural outcomes, as assessed by cognitive and locomotor tasks, as well as biochemical and histopathological outcomes, as assessed by neurotransmitter levels and the structure of neurovascular units. Although most clinical studies addressed stroke, the literature remains inconclusive.
There are several interlinked themes that have emerged regarding the mechanism of how SGLT2i may benefit neurological disorders (Figure 2). First, SGLT2i play an important role in mitigating common risk factors for these disorders at the organism level, such as persistent hyperglycaemia, insulin resistance, high triglyceride levels, visceral fat and hypertension. 66 SGLT2i lower blood pressure possibly through the osmotic and diuretic effects and reduction in sodium reabsorption by the kidneys. Hypertension is a well-known risk factor for stroke and dementia as it leads to endothelial dysfunction, atherosclerosis and small vessel disease, and reduced blood pressure is associated with lower risk of stroke and TIA. 67 A reduction in circulating plasma volume through natriuresis and diuresis and associated haemoconcentration is proposed to explain the improvement in heart failure outcomes associated with SGLT2i, 68 which may reduce the hypoperfusion associated with heart failure, improving cerebral blood flow, and reducing the risk of stroke. These risk factors are relevant not only to stroke, but also to neurodegenerative disorders, where metabolic dysfunctions such as insulin resistance, impaired insulin-like growth factor-1 signalling, impaired beta-cell function and altered leptin and ghrelin levels are evident. 69
Figure 2.
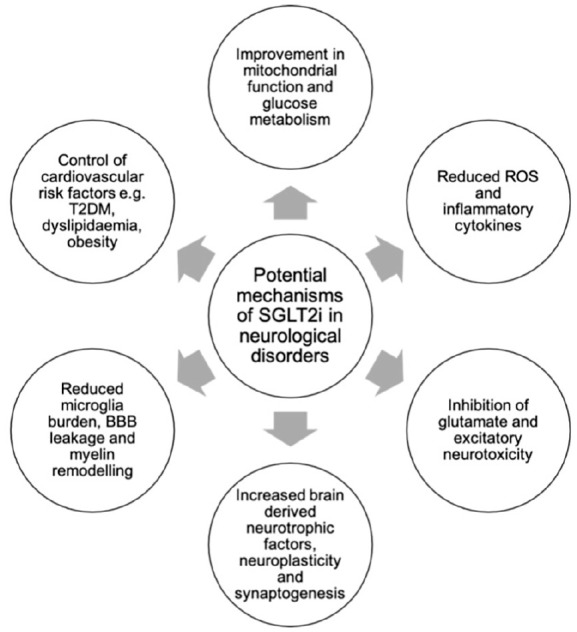
Various mechanisms via which SGLT2i may exert their beneficial effects on neurological disorders.
Second, the anti-inflammatory and anti-apoptotic effects of SGLT2i counteract the neuroinflammation observed in numerous disorders. For example, there is mounting evidence for a reciprocal relationship between local inflammation and the senile plaques and neurofibrillary tangles in AD, mediated by microglia and astrocytes. 70 Oxidative stress, lipid homeostasis dysfunction and other contributors to inflammation also display a reciprocal relationship with endoplasmic reticulum stress, leading to chronic overaction of the unfolded protein response: a fundamental process in the protein misfolding and apoptotic neuronal cell death observed in neurodegenerative disorders. 71 Similarly, mitochondrial dysfunction has been found in the substantia nigra of PD patients, leading to the formation of ROS, further propelling the inflammatory cascades seen in this disorder. 72 Moreover, inflammatory cytokines potentiate free radical species, altering glutamatergic neurotransmission and ultimately resulting in the neuronal excitotoxicity seen in epilepsy. 73 In addition to limiting excitotoxicity, SGLT2i also enhanced synaptogenesis and angiogenesis, contributing to neuroplasticity: 16 a mechanism essential in neurorehabilitation. At the cellular level, SGLT2i improves vascular function by inducing endothelium-independent vasorelaxation, reducing vascular adhesion molecule expression and macrophage vessel wall infiltration, thus reducing atherogenesis. 74 SGLT2i is also shown to reduce vascular inflammation through AMP-activated protein kinase-dependent and independent mechanisms in animal studies, and reduces arterial stiffness via anti-inflammatory mechanisms, which may contribute to the reduction in stroke. 75 Overall, the anti-inflammatory and antioxidant effects of SGLT2i lend further support for their potential in a range of neurological disorders.
The third theme is the ketogenic effects of SGLT2i; ketogenesis is significantly augmented by SGLT2i and is an effect that is often noted in previous literature. 28 The therapeutic use of the ketogenic diet in neurological disorders is well established, especially for paediatric epilepsy syndromes such as West syndrome. 76 Ketosis has shown improvement in the cognitive function of patients with AD, 10 and in animal models of traumatic brain injury through limiting cerebral oedema and contusion volume while increasing tissue sparing. 77 On a molecular level, these effects are thought to be mediated by enhanced mitochondrial respiration through peroxisome proliferator–activated receptors (PPARs) and AMPK activation. 77 SGLT2i also reduce advanced glycation end products (AGEs) and their receptor (RAGE) interactions, which are products of non-enzymatic glycation of macromolecules associated with hyperglycaemia in diabetes, aging and inflammation. They increase inflammation and oxidative stress, causing extracellular damage in organs such as kidneys, leading to diabetic nephropathy, and diabetic vascular complications including stroke. 78 Empagliflozin is shown to inhibit oxidative, inflammatory and fibrotic reactions in the kidney partially via the suppression of the AGE–RAGE axis, but the impact on stroke needs further study. 79
The pleiotropic effects of SGLT2i are not without risks, and SGLT2i are associated with adverse events that may have neurological consequences. Canagliflozin, dapagliflozin and empagliflozin are consistently associated with increased genital tract infection compared with placebo and other antiglycaemic agents. 80 Only dapagliflozin was associated with increased urinary tract infection, while both canagliflozin and dapagliflozin were associated with diabetes ketoacidosis (DKA). 80 Cerebral oedema is the most severe neurological complication associated with DKA, causing chronic central nervous system morbidity in 10–25% of affected children, such as poor delayed memory recall and divided attention at 6 months. 81 SGLT2i, although less likely to cause hypoglycaemia than insulin, are associated with increased hypoglycaemia when used in combination with metformin and sulfonylureas. 80 Neurological sequelae of hypoglycaemia range from full recovery to persistent vegetative state, and manifestations include confusion, seizures, focal neurological deficits and coma. 82 Therefore, although generally safe, the neurological adverse events of SGLT2i cannot be overlooked.
Given the favourable effects of SGLT2i demonstrated in animal studies, their ability to cross the BBB and their action on multiple receptors in docking studies, this group of antidiabetic drugs should be an essential part of future neurological trials. At the time of writing, a randomised controlled trial is ongoing (NCT03801642), investing the effect on dapagliflozin in patients with AD, which will be an important basis for the further trials on the impact of SGLT2i on neurological disorders.
Strengths and limitations
A key strength of this review stems from its novelty; this is the first scoping review to investigate the potential of SGLT2i in neurological disorders through a systematic search and analysis of 160 studies. Furthermore, our review collates the data from pre-clinical and clinical studies alike, and analyses the various mechanisms postulated in these studies. The consistency of benefits found in the range of studies included strengthens the argument that SGLT2i may exhibit neuroprotective potential. However, it is important to note the paucity of human studies. This may be because current clinical trials are specifically powered for diabetic, renal or cardiovascular outcomes of SGLT2i. Consequently, clinical analysis was based on stroke data extracted from cardiovascular outcome trials, or individual case reports, which cannot be regarded as a source of evidence-based conclusions. Due to the lack of evidence from clinical trials and large cohort studies, quantitative analysis was not possible in this systematic review.
Second, clinical trials were heterogeneous with respect to the interventions, comparators and populations investigated. Several studies used a combination of drugs alongside SGLT2i, either placebo or other antidiabetic medications were used as comparators, and the population of interest varied from healthy participants to individuals affected by diabetes or chronic kidney disease. Those who had comorbidities may have worse neurological outcomes from the start and subsequently experience the greatest benefit from SGLT2i, presenting a ceiling effect. This heterogeneity in included studies made it challenging to delineate the most accurate effect of SGLT2i on neurological outcomes.
Third, most studies looked at pre-treatment with SGLT2i, rather than the use of SGLT2i after the neurological event. It can, therefore, be inferred that while SGLT2i may be helpful in primary prevention, their utility in secondary or tertiary prevention is less certain. On the contrary, some studies suggest that the post-stroke use of SGLT2i can enhance neuronal synaptic plasticity and limit cerebral oedema.8,63 Future studies can further clarify whether SGLT2i are indeed useful in secondary and tertiary prevention.
In this review, we focused on SGLT2 inhibition by SGLT2-selective inhibitors, but SLGT1 inhibition may have an additional role in the neurological effects of SGLT inhibition. The brain expression of SGLT1 is higher than SGLT2, and SGLT1 is mainly found in the pyramidal cells of the cortex, Purkinje cerebellum cells, hippocampus pyramidal and granular cells and hypothalamus. 83 Phlorizin is a non-selective SGLT inhibitor, which is found to reverse post-ischaemic cerebral hyperglycaemia and cerebral oedema in stroke. 8 Conversely, phlorizin is also associated with increased severity of neurodegenerative process post-seizures, possibly due to less glucose entry into the cells with reduced ATP generation, triggering cell death molecular cascades resulting in neurodegeneration. 29 The effects of SGLT1 versus SGLT2 inhibition in neuroprotection require further investigation and delineation, which is of increasing clinical relevance due to the availability of SGLT1/2 dual inhibitors, such as sotagliflozin, on the market. 84
Conclusion
SGLT2 inhibitors exhibit promising potential for the management of neurological diseases, ranging from acute stroke to chronic problems such as AD, epilepsy and PD. The beneficial effects are related to their anti-inflammatory, antioxidant and ketogenic effects on a microscopic scale, which is reflected in improved neurovascular structure and cognitive outcomes on a macroscopic scale. Given the increasing prevalence of neurological diseases in the context of an aging population, there is a dire need to consider repurposing existing medications on the market. Although animal studies and docking studies demonstrate that these antidiabetic medications can have beneficial effects in neurological diseases, it is important to further explore their potential in human populations through future research.
Supplemental Material
Supplemental material, sj-docx-1-taj-10.1177_20406223221086996 for Sodium-glucose cotransporter 2 inhibitors and neurological disorders: a scoping review by Thahesh Tharmaraja, Jamie S.Y. Ho, Ching-Hui Sia, Nicole-Ann Lim, Yao Feng Chong, Amanda Y.L. Lim, Rahul R. Rathakrishnan, Leonard L.L. Yeo, Vijay K. Sharma and Benjamin Y.Q. Tan in Therapeutic Advances in Chronic Disease
Acknowledgments
Dr Benjamin YQ Tan is supported by the Ministry of Health, Singapore (MOH) Healthcare Research Scholarship – Master of Clinical Investigation (MCI) and the Chan Heng Leong Research Award.
Footnotes
Author contributions: Thahesh Tharmaraja: Data curation; Formal analysis; Investigation; Methodology; Visualisation; Writing – original draft; Writing – review & editing.
Jamie S.Y. Ho: Formal analysis; Investigation; Methodology; Visualisation; Writing – original draft; Writing – review & editing.
Ching-Hui Sia: Conceptualisation; Methodology; Project administration; Supervision; Validation.
Nicole-Ann Lim: Writing – review & editing.
Yao Feng Chong: Writing – review & editing.
Amanda Y.L. Lim: Writing – review & editing.
Rahul R. Rathakrishnan: Writing – review & editing.
Leonard L.L. Yeo: Writing – review & editing.
Vijay K. Sharma: Writing – review & editing.
Benjamin Y.Q. Tan: Conceptualisation; Methodology; Project administration; Supervision; Validation.
Data sharing: All authors confirm that all relevant data have been included in this paper, appendices and supplementary information files.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iD: Thahesh Tharmaraja  https://orcid.org/0000-0003-3023-2577
https://orcid.org/0000-0003-3023-2577
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Thahesh Tharmaraja, Intensive Care Unit, University College Hospital, University College London Hospitals NHS Foundation Trust, London, UK.
Jamie S.Y. Ho, Intensive Care Unit, Royal Free Hospital, Royal Free London NHS Foundation Trust, London, UK.
Ching-Hui Sia, Department of Medicine, Yong Loo Lin School of Medicine, National University of Singapore, Singapore.
Nicole-Ann Lim, Department of Medicine, Yong Loo Lin School of Medicine, National University of Singapore, Singapore.
Yao Feng Chong, Division of Neurology, Department of Medicine, National University Health System, Singapore.
Amanda Y.L. Lim, Division of Endocrinology, Department of Medicine, National University Health System, Singapore
Rahul R. Rathakrishnan, Division of Neurology, Department of Medicine, National University Health System, Singapore
Leonard L.L. Yeo, Division of Neurology, Department of Medicine, National University Health System, Singapore; Department of Medicine, Yong Loo Lin School of Medicine, National University of Singapore, NUHS Tower Block, 1E Kent Ridge Road Level 11, 119228 Singapore.
Vijay K. Sharma, Department of Medicine, Yong Loo Lin School of Medicine, National University of Singapore, Singapore; Division of Neurology, Department of Medicine, National University Health System, Singapore
Benjamin Y.Q. Tan, Department of Medicine, Yong Loo Lin School of Medicine, National University of Singapore, Singapore; Division of Neurology, Department of Medicine, National University Health System, Singapore
References
- 1. Teo YH, Teo YN, Syn NL, et al. Effects of sodium/glucose cotransporter 2 (SGLT2) inhibitors on cardiovascular and metabolic outcomes in patients without diabetes mellitus: a systematic review and meta-analysis of randomized-controlled trials. J Am Heart Assoc 2021; 10: e019463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gorelick PB. The global burden of stroke: persistent and disabling. Lancet Neurol 2019; 18: 417–418. [DOI] [PubMed] [Google Scholar]
- 3. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2019; 18: 88–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet 2015; 385: 2255–2263. [DOI] [PubMed] [Google Scholar]
- 5. Oskarsson ME, Paulsson JF, Schultz SW, et al. In vivo seeding and cross-seeding of localized amyloidosis: a molecular link between type 2 diabetes and Alzheimer disease. Am J Pathol 2015; 185: 834–846. [DOI] [PubMed] [Google Scholar]
- 6. Erdogan MA, Yusuf D, Christy J, et al. Highly selective SGLT2 inhibitor dapagliflozin reduces seizure activity in pentylenetetrazol-induced murine model of epilepsy. BMC Neurol 2018; 18: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Amin EF, Rifaai RA, Abdel-Latif RG. Empagliflozin attenuates transient cerebral ischemia/reperfusion injury in hyperglycemic rats via repressing oxidative–inflammatory–apoptotic pathway. Fundam Clin Pharmacol 2020; 34: 548–558. [DOI] [PubMed] [Google Scholar]
- 8. Yamazaki Y, Harada S, Tokuyama S. Post-ischemic hyperglycemia exacerbates the development of cerebral ischemic neuronal damage through the cerebral sodium-glucose transporter. Brain Res 2012; 1489: 113–120. [DOI] [PubMed] [Google Scholar]
- 9. D’Andrea Meira I, Romão TT, Pires do, Prado HJ, et al. Ketogenic diet and epilepsy: what we know so far. Front Neurosci 2019; 13: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Henderson ST, Vogel JL, Barr LJ, et al. Study of the ketogenic agent AC-1202 in mild to moderate Alzheimer’s disease: a randomized, double-blind, placebo-controlled, multicenter trial. Nutr Metab 2009; 6: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Phillips MCL, Murtagh DKJ, Gilbertson LJ, et al. Low-fat versus ketogenic diet in Parkinson’s disease: a pilot randomized controlled trial. Mov Disord 2018; 33: 1306–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Parker WD, Jr, Parks JK, Swerdlow RH. Complex I deficiency in Parkinson’s disease frontal cortex. Brain Res 2008; 1189: 215–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 2018; 169: 467–473. [DOI] [PubMed] [Google Scholar]
- 14. Zilliox LA, Chadrasekaran K, Kwan JY, et al. Diabetes and cognitive impairment. Curr Diab Rep 2016; 16: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Arafa NMS, Marie MAS, AlAzimi SAM. Effect of canagliflozin and metformin on cortical neurotransmitters in a diabetic rat model. Chem Biol Interact 2016; 258: 79–88. [DOI] [PubMed] [Google Scholar]
- 16. Wang S, Fan F. Oral antihyperglycemic therapy with a SGLT2 inhibitor reverses cognitive impairments in elderly diabetics. Hypertension 2019; 74: A051. [Google Scholar]
- 17. Millar P, Pathak N, Parthsarathy V, et al. Metabolic and neuroprotective effects of dapagliflozin and liraglutide in diabetic mice. J Endocrinol 2017; 234: 255–267. [DOI] [PubMed] [Google Scholar]
- 18. Hayden MR, Grant DG, Aroor AR, et al. Empagliflozin ameliorates type 2 diabetes-induced ultrastructural remodeling of the neurovascular unit and neuroglia in the female db/db mouse. Brain Sci 2019; 9: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hierro-Bujalance C, Infante-Garcia C, Del Marco A, et al. Empagliflozin reduces vascular damage and cognitive impairment in a mixed murine model of Alzheimer’s disease and type 2 diabetes. Alzheimers Res Ther 2020; 12: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rani R, Kumar A, Jaggi AS, et al. Pharmacological investigations on efficacy of Phlorizin a sodium-glucose co-transporter (SGLT) inhibitor in mouse model of intracerebroventricular streptozotocin induced dementia of AD type. J Basic Clin Physiol Pharmacol 2021; 32: 1057–1064. [DOI] [PubMed] [Google Scholar]
- 21. Panchal S, Chhabra S, Prasad BK, et al. Management of cognitive decline in T2DM – SGLT2 inhibitors at horizon. Indian J Endocrinol Metab 2018; 22: S28. [Google Scholar]
- 22. Shaikh S, Rizvi SM, Shakil S, et al. Forxiga (dapagliflozin): plausible role in the treatment of diabetes-associated neurological disorders. Biotechnol Appl Biochem 2016; 63: 145–150. [DOI] [PubMed] [Google Scholar]
- 23. Rizvi SM, Shakil S, Biswas D, et al. Invokana (Canagliflozin) as a dual inhibitor of acetylcholinesterase and sodium glucose co-transporter 2: advancement in Alzheimer’s disease- diabetes type 2 linkage via an enzoinformatics study. CNS Neurol Disord Drug Targets 2014; 13: 447–451. [DOI] [PubMed] [Google Scholar]
- 24. Alafnan A. Biochemical interaction analysis of natural SGLT2 inhibitors with Alzheimer targets: a computational approach. J Biochem Technol 2020; 11: 73–84. [Google Scholar]
- 25. Wium-Andersen IK, Osler M, Jørgensen MB, et al. Antidiabetic medication and risk of dementia in patients with type 2 diabetes: a nested case-control study. Eur J Endocrinol 2019; 181: 499–507. [DOI] [PubMed] [Google Scholar]
- 26. Perna S, Mainardi M, Astrone P, et al. 12-month effects of incretins versus SGLT2-inhibitors on cognitive performance and metabolic profile. A randomized clinical trial in the elderly with type-2 diabetes mellitus. Clin Pharmacol 2018; 10: 141–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Unno K, Takagi Y, Konishi T, et al. Mutation in sodium-glucose cotransporter 2 results in down-regulation of amyloid beta (A4) precursor-like protein 1 in young age, which may lead to poor memory retention in old age. Int J Mol Sci 2020; 21: 5579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Blunck JR, Newman JW, Fields RK, et al. Therapeutic augmentation of ketogenic diet with a sodium-glucose cotransporter 2 inhibitor in a super-refractory status epilepticus patient. Epilepsy Behav Case Rep 2018; 10: 61–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Melo IS, Santos YMO, Costa MA, et al. Inhibition of sodium glucose cotransporters following status epilepticus induced by intrahippocampal pilocarpine affects neurodegeneration process in hippocampus. Epilepsy Behav 2016; 61: 258–268. [DOI] [PubMed] [Google Scholar]
- 30. Melo IS, Santos YMO, Pacheco ALD, et al. Functional analysis of phlorizin in brain plasticity after status epilepticus induced intrahippocampal microinjection of pilocarpine. Epileptic Disord 2014; 16: 96–97.24691301 [Google Scholar]
- 31. Anandhan A, Lei S, Levytskyy R, et al. Glucose metabolism and AMPK signaling regulate dopaminergic cell death induced by gene (α-synuclein)-environment (paraquat) interactions. Mol Neurobiol 2017; 54: 3825–3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Arab HH, Safar MM, Shahin NN. Targeting ROS-dependent Akt/GSK-3β/NF-κB and DJ-1/Nrf2 pathways by dapagliflozin attenuates neuronal injury and motor dysfunction in rotenone-induced Parkinson’s disease rat model. ACS Chem Neurosci 2021; 12: 689–703. [DOI] [PubMed] [Google Scholar]
- 33. Sheth S, Brito R, Mukherjea D, et al. Adenosine receptors: expression, function and regulation. Int J Mol Sci 2014; 15: 2024–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pinna A. Adenosine A2A receptor antagonists in Parkinson’s disease: progress in clinical trials from the newly approved istradefylline to drugs in early development and those already discontinued. CNS Drugs 2014; 28: 455–474. [DOI] [PubMed] [Google Scholar]
- 35. Ayoub BM, Michel HE, Mowaka S, et al. Repurposing of omarigliptin as a neuroprotective agent based on docking with A2A adenosine and AChE receptors, brain GLP-1 response and its brain/plasma concentration ratio after 28 days multiple doses in rats using LC-MS/MS. Molecules 2021; 26: 889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Paton DM. Istradefylline: adenosine A2A receptor antagonist to reduce ‘OFF’ time in Parkinson’s disease. Drugs Today 2020; 56: 125–134. [DOI] [PubMed] [Google Scholar]
- 37. El-Sahar AE, Rastanawi AA, El-Yamany MF, et al. Dapagliflozin improves behavioral dysfunction of Huntington’s disease in rats via inhibiting apoptosis-related glycolysis. Life Sci 2020; 257: 118076. [DOI] [PubMed] [Google Scholar]
- 38. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019; 380: 347–357. [DOI] [PubMed] [Google Scholar]
- 39. Cannon CP, Pratley R, Dagogo-Jack S, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med 2020; 383: 1425–1435. [DOI] [PubMed] [Google Scholar]
- 40. Katakami N, Mita T, Yoshii H, et al. Tofogliflozin does not delay progression of carotid atherosclerosis in patients with type 2 diabetes: a prospective, randomized, open-label, parallel-group comparative study. Cardiovasc Diabetol 2020; 19: 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chen TH, Li YR, Chen SW, et al. Sodium-glucose cotransporter 2 inhibitor versus metformin as first-line therapy in patients with type 2 diabetes mellitus: a multi-institution database study. Cardiovasc Diabetol 2020; 19: 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Goh SY, Kim DJ, Lam C, et al. Lower risk of cardiovascular events and death associated with initiation of SGLT2 inhibitors vs. Sulphonylureas’ analysis from the CVD-REAL 2 study. J Diabetes Investig 2018; 9: 126. [DOI] [PubMed] [Google Scholar]
- 43. Korgaonkar S, Yang Y, Pitcock J, et al. PDB7 assessment of cardiovascular outcomes associated with sodium glucose cotransporter 2 (SGLT2) inhibitor therapy and incretin-based therapies among elderly patients type 2 diabetes mellitus. Value Health 2020; 23: S108–S109. [Google Scholar]
- 44. Patorno E, Goldfine AB, Schneeweiss S, et al. Cardiovascular outcomes associated with canagliflozin versus other non-gliflozin antidiabetic drugs: population based cohort study. BMJ 2018; 360: k119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Patorno E, Everett BM, Schneeweiss S, et al. Comparative cardiovascular effectiveness of SGLT2 inhibitors vs. Liraglutide in routine care. Diabetes 2018; 67: A399. [Google Scholar]
- 46. Patorno E, Pawar A, Bessette LG, et al. Effectiveness and safety of empagliflozin in routine care patients: interim results from the empagliflozin comparative effectiveness and safety (EMPRISE) study. Diabetes 2020; 69(Supplement_1): 134–LB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wong CKH, Tang EHM, Man KKC, et al. SGLT2i as fourth-line therapy and risk of mortality, end-stage renal diseases and cardiovascular diseases in patients with type 2 diabetes mellitus. Diabetes Metab 2020; 47: 101196. [DOI] [PubMed] [Google Scholar]
- 48. Pasternak B, Ueda P, Eliasson B, et al. Use of sodium glucose cotransporter 2 inhibitors and risk of major cardiovascular events and heart failure: Scandinavian register based cohort study. BMJ 2019; 366: l4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Norhammar A, Bodegård J, Nyström T, et al. Dapagliflozin and cardiovascular mortality and disease outcomes in a population with type 2 diabetes similar to that of the DECLARE-TIMI 58 trial: a nationwide observational study. Diabetes Obes Metab 2019; 21: 1136–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Goda M, Yamakura T, Sasaki K, et al. Safety and efficacy of canagliflozin in elderly patients with type 2 diabetes mellitus: a 1-year post-marketing surveillance in Japan. Curr Med Res Opin 2018; 34: 319–327. [DOI] [PubMed] [Google Scholar]
- 51. Kohsaka S, Lam CSP, Kim DJ, et al. Risk of cardiovascular events and death associated with initiation of SGLT2 inhibitors compared with DPP-4 inhibitors: an analysis from the CVD-REAL 2 multinational cohort study. Lancet Diabetes Endocrinol 2020; 8: 606–615. [DOI] [PubMed] [Google Scholar]
- 52. Persson F, Nyström T, Jørgensen ME, et al. Dapagliflozin is associated with lower risk of cardiovascular events and all-cause mortality in people with type 2 diabetes (CVD-REAL Nordic) when compared with dipeptidyl peptidase-4 inhibitor therapy: a multinational observational study. Diabetes Obes Metab 2018; 20: 344–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Shao SC, Chang KC, Hung MJ, et al. Comparative risk evaluation for cardiovascular events associated with dapagliflozin vs. Empagliflozin in real-world type 2 diabetes patients: a multi-institutional cohort study. Cardiovasc Diabetol 2019; 18: 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Qiu M, Ding LL, Zhang M, et al. GLP-1RAs and SGLT2is reduce cardiovascular events independent of reductions of systolic blood pressure and body weight: a meta-analysis with meta-regression. Diabetes Ther 2020; 11: 2429–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Barkas F, Ntekouan SF, Liberopoulos E, et al. Sodium-glucose cotransporter-2 inhibitors and protection against stroke in patients with type 2 diabetes and impaired renal function: a systematic review and meta-analysis. J Stroke Cerebrovasc Dis 2021; 30: 105708. [DOI] [PubMed] [Google Scholar]
- 56. Toyama T, Neuen BL, Jun M, et al. Effect of SGLT2 inhibitors on cardiovascular, renal and safety outcomes in patients with type 2 diabetes mellitus and chronic kidney disease: a systematic review and meta-analysis. Diabetes Obes Metab 2019; 21: 1237–1250. [DOI] [PubMed] [Google Scholar]
- 57. Aronow WS, Shamliyan TA. Comparative effectiveness and safety of empagliflozin on cardiovascular mortality and morbidity in adults with type 2 diabetes. Ann Transl Med 2017; 5: 455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Li CX, Liang S, Gao L, et al. Cardiovascular outcomes associated with SGLT-2 inhibitors versus other glucose-lowering drugs in patients with type 2 diabetes: a real-world systematic review and meta-analysis. PLoS ONE 2021; 16: e0244689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Abdel-Latif RG, Rifaai RA, Amin EF. Empagliflozin alleviates neuronal apoptosis induced by cerebral ischemia/reperfusion injury through HIF-1 alpha/VEGF signaling pathway. Arch Pharm Res 2020; 43: 514–525. [DOI] [PubMed] [Google Scholar]
- 60. Zhang W, Welihinda A, Mechanic J, et al. EGT1442, a potent and selective SGLT2 inhibitor, attenuates blood glucose and HbA(1c) levels in db/db mice and prolongs the survival of stroke-prone rats. Pharmacol Res 2011; 63: 284–293. [DOI] [PubMed] [Google Scholar]
- 61. Harada S, Yamazaki Y, Nishioka H, et al. Neuroprotective effect through the cerebral sodium-glucose transporter on the development of ischemic damage in global ischemia. Brain Res 2013; 1541: 61–68. [DOI] [PubMed] [Google Scholar]
- 62. Vemula S, Roder KE, Yang T, et al. A functional role for sodium-dependent glucose transport across the blood-brain barrier during oxygen glucose deprivation. J Pharmacol Exp Ther 2009; 328: 487–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yamazaki Y, Harada S, Tokuyama S. Relationship between cerebral sodium-glucose transporter and hyperglycemia in cerebral ischemia. Neurosci Lett 2015; 604: 134–139. [DOI] [PubMed] [Google Scholar]
- 64. Yamazaki Y, Harada S, Wada T, et al. Sodium transport through the cerebral sodium-glucose transporter exacerbates neuron damage during cerebral ischaemia. J Pharm Pharmacol 2016; 68: 922–931. [DOI] [PubMed] [Google Scholar]
- 65. Sriwichaiin S, Lahnwong S, Apaijai N, et al. Pretreatment with dapagliflozin provides neuroprotective effects following cardiac ischemic/reperfusion (I/R) injury by decreasing amyloid beta aggregation and blood-brain barrier breakdown. Alzheimers Dement 2019; 15: P303. [Google Scholar]
- 66. Al Hamed FA, Elewa H. Potential therapeutic effects of sodium glucose-linked cotransporter 2 inhibitors in stroke. Clin Ther 2020; 42: e242–e249. [DOI] [PubMed] [Google Scholar]
- 67. Zonneveld TP, Richard E, Vergouwen MDI, et al. Blood pressure-lowering treatment for preventing recurrent stroke, major vascular events, and dementia in patients with a history of stroke or transient ischaemic attack. Cochrane Database Syst Rev 2018; 7: CD007858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117–2128. [DOI] [PubMed] [Google Scholar]
- 69. Cai H, Cong WN, Ji S, et al. Metabolic dysfunction in Alzheimer’s disease and related neurodegenerative disorders. Curr Alzheimer Res 2012; 9: 5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zilka N, Ferencik M, Hulin I. Neuroinflammation in Alzheimer’s disease: protector or promoter? Bratisl Lek Listy 2006; 107: 374–383. [PubMed] [Google Scholar]
- 71. Cao SS, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid Redox Signal 2014; 21: 396–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Keane PC, Kurzawa M, Blain PG, et al. Mitochondrial dysfunction in Parkinson’s disease. Parkinsons Dis 2011; 2011: 716871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kobylarek D, Iwanowski P, Lewandowska Z, et al. Advances in the potential biomarkers of epilepsy. Front Neurol 2019; 10: 685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Gaspari T, Spizzo I, Liu H, et al. Dapagliflozin attenuates human vascular endothelial cell activation and induces vasorelaxation: a potential mechanism for inhibition of atherogenesis. Diab Vasc Dis Res 2018; 15: 64–73. [DOI] [PubMed] [Google Scholar]
- 75. Mancini SJ, Boyd D, Katwan OJ, et al. Canagliflozin inhibits interleukin-1β-stimulated cytokine and chemokine secretion in vascular endothelial cells by AMP-activated protein kinase-dependent and -independent mechanisms. Sci Rep 2018; 8: 5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wheless JW. Managing severe epilepsy syndromes of early childhood. J Child Neurol 2009; 24(Suppl. 8): 24S–32S; quiz 3S–6S. [DOI] [PubMed] [Google Scholar]
- 77. Gano LB, Patel M, Rho JM. Ketogenic diets, mitochondria, and neurological diseases. J Lipid Res 2014; 55: 2211–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Egaña-Gorroño L, López-Díez R, Yepuri G, et al. Receptor for advanced glycation end products (RAGE) and mechanisms and therapeutic opportunities in diabetes and cardiovascular disease: insights from human subjects and animal models. Front Cardiovasc Med 2020; 7: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ojima A, Matsui T, Nishino Y, et al. Empagliflozin, an inhibitor of sodium-glucose cotransporter 2 exerts anti-inflammatory and antifibrotic effects on experimental diabetic nephropathy partly by suppressing AGEs-receptor axis. Horm Metab Res 2015; 47: 686–692. [DOI] [PubMed] [Google Scholar]
- 80. Pelletier R, Ng K, Alkabbani W, et al. Adverse events associated with sodium glucose co-transporter 2 inhibitors: an overview of quantitative systematic reviews. Ther Adv Drug Saf 2021; 12: 2042098621989134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Cameron FJ, Scratch SE, Nadebaum C, et al. Neurological consequences of diabetic ketoacidosis at initial presentation of type 1 diabetes in a prospective cohort study of children. Diabetes Care 2014; 37: 1554–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Barbara G, Mégarbane B, Argaud L, et al. Functional outcome of patients with prolonged hypoglycemic encephalopathy. Ann Intensive Care 2017; 7: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Pawlos A, Broncel M, Woźniak E, et al. Neuroprotective effect of SGLT2 inhibitors. Molecules 2021; 26: 7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Bhatt DL, Szarek M, Steg PG, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med 2020; 384: 117–128. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-taj-10.1177_20406223221086996 for Sodium-glucose cotransporter 2 inhibitors and neurological disorders: a scoping review by Thahesh Tharmaraja, Jamie S.Y. Ho, Ching-Hui Sia, Nicole-Ann Lim, Yao Feng Chong, Amanda Y.L. Lim, Rahul R. Rathakrishnan, Leonard L.L. Yeo, Vijay K. Sharma and Benjamin Y.Q. Tan in Therapeutic Advances in Chronic Disease