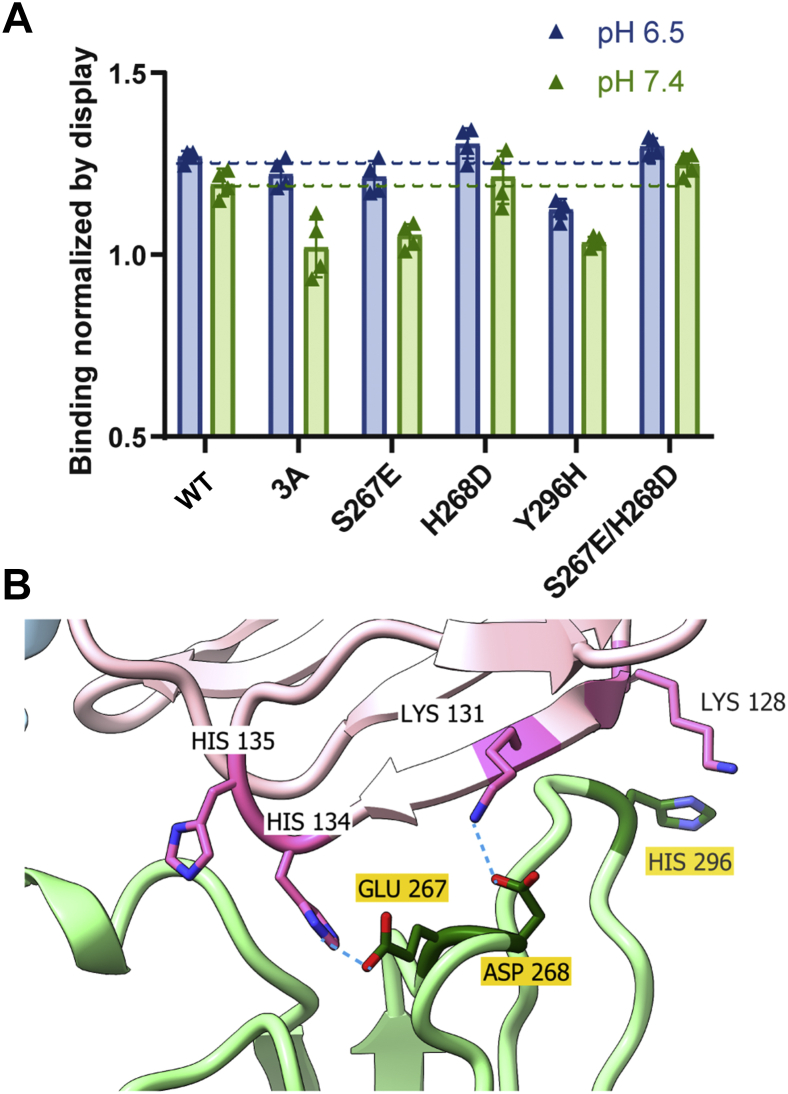
Figure 7.
Contributions of acid-Fc residue changes to pH selectivity.A, the three residues altered in acid-Fc were introduced into wild-type (WT) Fc in various combinations and displayed on the CHO cell surface alongside control Fc domains before staining to detect specific FcγRIIIa binding at pH 6.5 and 7.4 by flow cytometry. Specific FcγRIIIa binding was determined as the percent of cells positive for binding to monomeric FcγRIIIa (V158) at 50 nM divided by the percent of cells positive for Fc display. The data shown represent the mean and SDs of two experimental repeats, each performed in duplicate. B, possible structural mechanism by which acid-Fc changes mediate pH selectivity. The Fc/FcγRIIIa structure (PDB 3SGJ) was modified to depict the acid-Fc residue changes using the most common rotamer in ChimeraX. Under acidic conditions, Fc S267E may form electrostatic interactions with protonated H134 on FcγRIIIa, while at both pH values, binding strength may be tuned by a salt bridge formed between Fc H268D and K131 on FcγRIIIa and Fc Y296H disruption of interactions with K128 on FcγRIIIa. CHO, chinese hamster ovary.