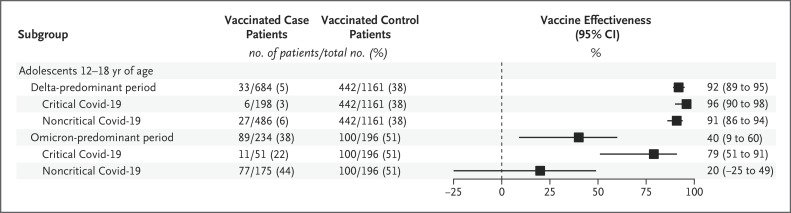
Figure 3. Effectiveness of the BNT162b2 Vaccine against Hospitalization for Critical as Compared with Noncritical Covid-19 in Adolescents 12 to 18 Years of Age, Stratified According to Variant.
Numbers were insufficient to stratify the analysis according to disease severity among children 5 to 11 years of age. In this analysis, only subgroups of case patients were based on disease severity; the entire control group (regardless of disease severity) served as the basis for comparison. Critical Covid-19 was defined as Covid-19 leading to life support (i.e., noninvasive mechanical ventilation [bilevel positive airway pressure or continuous positive airway pressure] or invasive mechanical ventilation, vasoactive infusions, or extracorporeal membrane oxygenation) or death. Information on this outcome was missing for 8 case patients admitted during the omicron period. Vaccine effectiveness was calculated as (1−adjusted odds ratio)×100, where the odds ratio is the odds of vaccination in case patients as compared with controls.