Abstract
Introduction
Population-wide interventions offer a pathway to tuberculosis (TB) and leprosy elimination, but ‘real-world’ implementation in a high-burden setting using a combined approach has not been demonstrated. This implementation study aims to demonstrate the feasibility and evaluate the effect of population-wide screening, treatment and prevention on TB and leprosy incidence rates, as well as TB transmission.
Methods and analysis
A non-randomised ‘screen-and-treat’ intervention conducted in the Pacific atoll of South Tarawa, Kiribati. Households are enumerated and all residents ≥3 years, as well as children <3 years with recent household exposure to TB or leprosy, invited for screening. Participants are screened using tuberculin skin testing, signs and symptoms of TB or leprosy, digital chest X-ray with computer-aided detection and sputum testing (Xpert MTB/RIF Ultra). Those diagnosed with disease are referred to the National TB and Leprosy Programme for management. Participants with TB infection are offered TB preventive treatment and those without TB disease or infection, or leprosy, are offered leprosy prophylaxis. The primary study outcome is the difference in the annual TB case notification rate before and after the intervention; a similar outcome is included for leprosy. The effect on TB transmission will be measured by comparing the estimated annual risk of TB infection in primary school children before and after the intervention, as a co-primary outcome used for power calculations. Comparison of TB and leprosy case notification rates in South Tarawa (the intervention group) and the rest of Kiribati (the control group) before, during and after the intervention is a secondary outcome.
Ethics and dissemination
Approval was obtained from the University of Sydney Human Research Ethics Committee (project no. 2021/127) and the Kiribati Ministry of Health and Medical Services (MHMS). Findings will be shared with the MHMS and local communities, published in peer-reviewed journals and presented at international conferences.
Keywords: Tuberculosis, EPIDEMIOLOGY, Epidemiology, Public health, Infection control, PREVENTIVE MEDICINE
Strengths and limitations of this study.
Real-world population-wide active case finding and prevention approach for tuberculosis (TB) and leprosy control.
Concurrent screening and treatment of TB disease and infection for durable public health impact.
Combined TB and leprosy elimination efforts increases efficiency, but also complexity.
Findings generalisable to other remote settings, but uncertain about ‘disease hot spots’ globally.
Non-randomised study design limits confidence in attribution of effects to the intervention.
Introduction
Tuberculosis (TB, caused by Mycobacterium tuberculosis (MTB)) is a leading infectious disease killer globally and in the Pacific,1 while leprosy (caused by Mycobacterium leprae) elimination remains a major challenge as well.2 It is estimated that TB killed ~1.5 million people in 2020, despite being a preventable and curable disease.1 At the 2018 United Nations High-Level Meeting on TB,3 world leaders committed to step up TB elimination efforts, with improved TB case finding and expanded use of TB preventive treatment (TPT) identified as key interventions in the global ‘End TB strategy’.4
Historical population-wide TB elimination programmes demonstrated considerable success and are enjoying renewed attention, with specific emphasis on active case finding and prevention strategies.5 6 A randomised controlled trial in Vietnam recently demonstrated that population-wide active TB case finding can achieve substantial reductions in TB incidence and transmission.7 Historic studies and modelling projections suggest that this effect, and its durability, can be enhanced by concurrent treatment of TB infection,8 9 but data on ‘real-life’ implementation remain scarce. Given WHO endorsement of integrated active TB case finding and preventive treatment approaches,10–15 there is urgency to develop implementation models that demonstrate feasibility and population impact.
At the 2020 census, around 59% of the i-Kiribati population lived on the capital atoll of Tarawa.16 Of all TB cases detected in Kiribati in 2020, 69% (267 of 385) were from Tarawa—equating to an estimated TB incidence of >500/100 000 population, the threshold incidence level above which the WHO recommends population-wide screening interventions.17 MTB strain typing data18 and the dominance of young adults among TB cases indicate high levels of community transmission,19 which emphasises the need for case finding and prevention strategies to be combined in order to prevent rapid reinfection.
Leprosy rates in Kiribati, and South Tarawa in particular, are also among the highest in the world (>200 cases/100 000 population, compared with the WHO elimination threshold of 10/100 000 population) and have been rising rapidly after a nadir in the late 1990s when elimination efforts were discontinued.2 Modelling studies done in Kiribati indicate that a single population-wide active case finding intervention, combined with ‘universal leprosy prophylaxis’ using single-dose rifampicin (SDR), could dramatically decrease leprosy prevalence with a sustained effect.20
Although both TB and leprosy pose daunting public health challenges in this resource-limited setting, their co-occurrence within a geographically defined and isolated population presents a unique opportunity to evaluate combined elimination efforts. The aim of the proposed study is to achieve major reductions in TB and leprosy incidence and transmission in South Tarawa over the medium term, providing a pathway to future elimination. If successful, the proposed study will provide a template for TB and leprosy elimination efforts throughout the Pacific and in other remote settings.
Methods and analysis
Study design
The Pathway to the Elimination of Antibiotic Resistant and Latent tuberculosis in the Pacific (PEARL) study is a before and after evaluation of a population-wide systematic screening intervention, combined with a comprehensive treatment and prevention programme for both TB and leprosy. It is executed in close collaboration with the Kiribati Ministry of Health and Medical Services (MHMS).
Setting
Kiribati is a lower-middle-income country of ~120 000 people residing on low-lying atolls and islands in the western Pacific Ocean, with the lowest gross domestic product per-capita of any country in Oceania.21 The country has among the highest TB incidence rates in the world,22 and is one of 23 WHO priority-countries for leprosy control.23 Small numbers of people have been diagnosed with drug-resistant (DR-) TB (7 out of 385 cases in 2020) with limited evidence of community transmission of DR-TB. The prevalence of HIV infection is low, and no cases of TB/HIV coinfection were reported in 2019. However, there is a high burden of non-communicable disease, with the adult diabetes prevalence exceeding 20%,24 high rates of childhood malnutrition and substantial levels of smoke exposure from cooking fires and cigarette smoking.25
As of November 2021, no confirmed cases of COVID-19 have been recorded in Kiribati, and no mask-wearing or social distancing measures have been employed. However, these practices and protocols are in place if required, should the COVID-19 pandemic spread to Kiribati. A COVID-19 vaccination programme commenced in May 2021, which is on track to achieve full vaccination coverage of >90% of the adult population by early 2022. Future public health measures to control the spread of COVID-19 will be determined by the Kiribati MHMS based on ongoing assessment of the risk posed and according to the National COVID-19 preparatory and response plan, and the standard operating procedures in place (Kiribati MHMS, unpublished).
Tarawa atoll serves as the capital of Kiribati. Residents of villages in South Tarawa and the small communities of Buota and Abatao at the southernmost end of North Tarawa are included in the intervention and hereunder referred to as ‘South Tarawa’. South Tarawa accommodates ~59% of the i-Kiribati population and has the highest rates of TB and leprosy disease. Betio Islet in particular is the most densely populated area in the Pacific with an estimated population density of >15 000 people/km2.
TB case management is provided by the National TB Programme (NTP). TB diagnostics available in South Tarawa include smear microscopy, rapid genotypic testing and chest X-ray (CXR), but culture facilities are unavailable. Sputum samples can be sent to the regional reference laboratory in Adelaide, Australia, for culture and susceptibility testing. However, this is problematic due to logistical issues and high rates of bacterial overgrowth. Most people with TB are hospitalised during the first few weeks of treatment at the local government hospital (Betio and Tungaru hospitals). Household contact investigation is advised, but not universally implemented because of resource constraints. National guidelines recommend TPT for household contacts for whom TB disease has been excluded, but implementation and uptake are again inconsistent. Leprosy is diagnosed using clinical assessment and microscopy, with genotypic diagnosis available by sending punch biopsy samples to the regional reference laboratory in Christchurch, New Zealand. Curative treatment, occupational therapy and chronic disease management is provided by the National Leprosy Programme (NLP). Postexposure prophylaxis (PEP) of household contacts using SDR has been scaled up in recent years with the help of the Pacific Leprosy Foundation (PLF).2
Study population
The study population comprises all residents of South Tarawa. All adults, adolescents and children aged three and above are included (table 1). Children aged less than 3 years are included if they have documented household contact with someone who had TB (in the past 1 year), or leprosy (at any time since they were born). Household contact is defined as being between two people who have regular meals prepared in the same kitchen. Members of the study population are only excluded if they prefer not to participate.
Table 1.
Study inclusion and exclusion criteria
Inclusion
|
Exclusion
|
TB, tuberculosis.
Study intervention
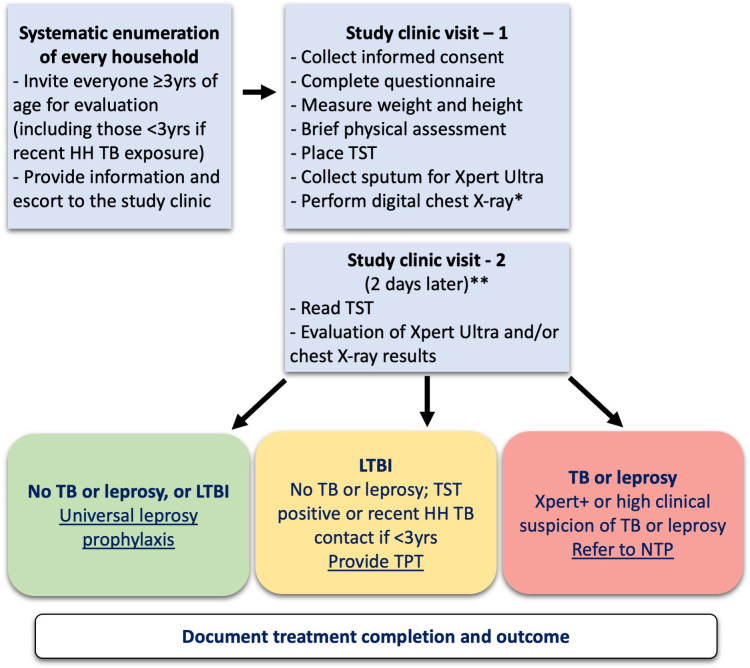
The study intervention comprises population-wide systematic screening and treatment of TB disease, TB infection and leprosy, together with the provision of leprosy prophylaxis to those not requiring treatment. In practice this equates to universal leprosy prophylaxis, given that TB and leprosy treatment, as well as TPT with rifamycin-based regimens, provides adequate leprosy prophylaxis. The enrolment and screening process are summarised in figure 1; more detailed algorithms are included in online supplemental files S1 and S2. The study intervention is delivered over a period of 3 years.
Figure 1.
Overview of study enrolment and population-wide TB and leprosy screening approachh. *Chest X-ray to be performed in everyone ≥10 years of age and all children <10 years with current symptoms (cough, fever, weight loss/failure to thrive or visible neck nodes). **The TST can be read up to 96 hours (4 days days) after placement if the 2-day day follow-up is missed. HH, household; LPT, leprosy preventive therapy; LTBI, latent TB infection; NTP, National TB Programme; TB, tuberculosis; TPT, TB preventive treatment; TST, tuberculosis;
bmjopen-2021-055295supp001.pdf (9.3MB, pdf)
Systematic screening procedures
All households are enumerated based on location and demographic data collected during the 2020 census (Kiribati National Statistics Office 2021, unpublished). Study staff visit each residence, collect GPS coordinates, ascertain eligibility in a brief household survey conducted with the household head, and invite eligible participants for screening. During the first clinic visit, study personnel obtain written informed consent from adult participants (≥18 years of age), or parents/legal guardians in those aged <18 years, with verbal assent from children aged 10–18 years. Identifying information is collected at registration, including a photograph and a biometric identifier to aid future re-identification (facial coordinate scan ‘health selfie’, Simprints Technology Limited, Cambridge, UK). Study nurses complete a short TB symptom questionnaire and a brief physical examination for signs and symptoms suggestive of TB disease (visible lymph node mass or gibbus) or leprosy (suggestive skin lesions or altered shape of face, nose, ears, hands or feet).
A tuberculin skin test (TST) is placed using the Mantoux method and sputum collection is attempted in all participants aged ≥10 years; age-appropriate diagnostic specimens may be collected in children <10 years who have symptoms suggestive of TB, but it is not part of the screening procedure. Sputum specimens are tested using Xpert MTB/RIF Ultra, recently endorsed by WHO as a sensitive front-line diagnostic test.26 A digital CXR is performed on everyone aged ≥10 years, and children aged <10 years with symptoms suggestive of TB. CXR interpretation is conducted with computer-aided detection (CAD) software certified for use in TB screening (CAD4TB v6, Diagnostic Image Analysis Group, Radboud University Medical Center, Nijmegen, The Netherlands). The use of CAD software for TB screening is recommended by recent WHO guidelines, and good experiences with implementation have been reported in a variety of settings.27 28 Screening CXRs for participants aged <15 years will also be interpreted by study medical staff, with the age threshold for CAD interpretation to be re-evaluated as evidence and experience accumulates during the intervention period.
At a second clinic visit 2 days later, the TST is read and considered positive if induration is ≥10 mm, or ≥5 mm if the participant has had household contact with a person with infectious TB in the past 12 months. The CXRs of all participants with a CAD score of ≥50 (and a random selection of those with scores <50), MTB detected on Xpert MTB/RIF Ultra or symptoms suggestive of TB will be reviewed by a study doctor. The CAD threshold score will be adjusted if necessary to optimise screening performance in the study population. Patients with bacteriologically confirmed or clinically diagnosed TB disease or leprosy will be referred to the Kiribati NTP or NLP for treatment, with close coordination between study and programme clinicians.
TPT eligibility
Participants with a positive TST or children <5 years with recent household contact are considered for TPT if they do not have TB disease and have not completed a course of TB treatment or TPT within the last 12 months. Potential TPT contraindications (eg, allergies to TB medicines, risk factors for hepatotoxicity) are assessed using a brief questionnaire. There is a high prevalence of hepatitis B virus (HBV) infection among older people in Kiribati29; routine HBV vaccination introduced in the 1990s reduced infection rates among younger people.30 A point-of-care HBV rapid test (Determine HBsAg 2) is performed in all participants aged 40–59 years who agree to TPT and are otherwise at low risk (liver function testing is done in everyone ≥60 years), since they are at increased risk of HBV infection and possible liver disease. Participants diagnosed with HBV infection receive a liver function test prior to TPT initiation and are referred to the HBV treatment programme for management.
Hepatotoxicity risk assessment
Baseline serum alanine aminotransferase (ALT) testing is conducted in all participants aged ≥60 years, or with identified risk factors for hepatotoxicity, and results stratified according to hepatotoxicity risk (table 2). Participants with ALT ≥3 x upper limit of normal (ULN) are not offered TPT, those with ALT 2–3 × ULN are offered TPT (if bilirubin <2 x ULN) with repeat ALT testing after 3–4 weeks, and participants with ALT <2 x ULN (or no need for a baseline test) are offered TPT without further ALT monitoring. All participants receive information on the signs and symptoms of hepatotoxicity, guidance to limit alcohol and kava intake and access to a ‘hotline’ linked to a rapid evaluation service.
Table 2.
TB preventive treatment eligibility and hepatotoxicity risk classification
| Assessment | Hepatotoxicity risk category | |||
| Low | Moderate | Moderate high | High | |
| Definition | ||||
Risk factors
|
No risk factors present | Any risk factor | Any risk factor | Any risk factor |
| ALT baseline test result (if any risk factors) | Not done | ALT<2 x ULN | ALT 2–3 x ULN and bilirubin <2xULN | ALT≥3 x ULN (or ALT 2–3 x ULN and bilirubin ≥2 x ULN) |
| Management | ||||
| Monitoring while on TPT† | No repeat ALT | No repeat ALT | Repeat ALT after 3–4 weeks | Not applicable |
| Eligibility for TPT | YES | YES | YES | NO |
| Other reasons for TPT ineligibility |
|
|||
*Everyone ≥40 years with a positive TST who agrees to TPT and is otherwise at low risk is tested with an HBV rapid antigen test.
†Everyone on TPT receives adherence and adverse event counselling, a TPT passport (online supplemental files S4 and S5), as well as access to community-based treatment monitoring and adherence support.
‡Excessive use’ defined as ≥3 days/week and/or (for alcohol) getting drunk every week.
ALT, alanine aminotransferase; HBV, hepatitis B virus; TB, tuberculosis; TPT, TB preventive treatment; TST, tuberculin skin test; ULN, upper limit of normal.
TPT regimens and treatment initiation
Participants who are eligible, have no contraindications and accept TPT, are offered a choice of short-course rifamycin-based regimens according to clinical characteristics, patient preference and availability. Currently used regimens are: 12 weekly doses of isoniazid and rifapentine (3HP), 4 months of daily rifampicin (4R) or 3 months of daily isoniazid and rifampicin (3RH, preferred for young children due to availability of child-friendly water-dispersible formulations). Dosing is chosen according to recommendations from the WHO and the Kiribati NTP. In participants with a documented history of household contact with someone diagnosed with DR-TB, TPT using 6 months of daily levofloxacin is considered under expert guidance.31 32 New evidence and normative guidance supporting the use of shorter TPT regimens may be released during the study. Consistent with the implementation approach, additional regimens may be offered to participants in collaboration with the Kiribati NTP and with updated ethical approval. All medicines used in the study will be obtained from WHO prequalified manufacturers, procured from the Global Drug Facility.
Community-based TPT adherence support and monitoring
At each stage of treatment initiation, adherence support, treatment monitoring, management of adverse events and assignment of treatment outcome, participants are provided with appropriate counselling on risks, benefits and options available. A detailed patient information sheet is provided (online supplemental file S3) together with group and/or individual counselling with the aid of an illustrated flip chart and a ‘TPT passport’ (online supplemental files S4 and S5) to assist adherence and adverse event monitoring. After taking the first dose under direct supervision, TPT will be self-administered with use of pragmatic adherence support strategies tailored to the particular study community. Medicines are dispensed in 4–8 weeks intervals, or according to patient preference and adherence. All participants who commence TPT are visited once after 3–4 weeks to support adherence and screen for adverse events, and again when they near TPT completion to assess treatment adherence and either extend treatment or assign a treatment outcome. Additional supportive visits are scheduled as needed. Assessment of adherence is performed and recorded at each visit by interviewing the participant, reviewing the TPT passport and counting remaining pills. Treatment completion is determined according to WHO recommendations (table 3).
Table 3.
Criteria for TB preventive treatment completion
| Regimen | Expected duration | Expected total doses | Minimum doses for completion | Maximum time for completion | TPT incomplete |
| 3HP (weekly) | 12 weeks | 12 | 11 | 16 weeks | <8 doses after 12 weeks |
| 3RH (daily) | 3 months | 84 | 68 | 4 months | <40 doses after 3 months |
| 4R (daily) | 4 months | 120 | 96 | 5 months | <68 doses after 4 months |
H, isoniazid; P, rifapentine; R, rifampicin; TB, tuberculosis; TPT, TB preventive treatment.
Detection and management of adverse events
This is an implementation study that uses medicines recommended by the Kiribati NTP and which have been shown to be safe and effective.10 33–38 Adverse events associated with the study intervention (defined as any untoward medical occurrence in a study participant) are similar to those encountered in routine programme delivery. Misidentification and misclassification will be minimised through adequate planning and testing of all procedures, using ongoing quality assurance measures built into a secure Research Electronic Data Capture (REDCap) database39 and close oversight from local study coordinators. Linkage to care for referrals to government treatment programmes (TB, leprosy, hepatitis) will be monitored.
Participants receiving TPT will have access to a team of community health workers, a ‘TPT hotline’, and a walk-in service for adverse event assessment and management. Study staff are trained to identify the most common and most severe adverse events associated with TPT, with a particular focus on hepatotoxicity. Drug-related adverse events are triaged and referred for further medical evaluation as appropriate, including liver function testing. If needed, referral and linkage to urgent care services is available.
The following drug-related adverse events will be recorded and reported as part of the study:
Drug-induced liver injury (DILI—defined as suggestive symptoms plus ALT >3 times ULN or ALT >5 times ULN without symptoms) while on TPT.
Drug-related adverse events resulting in TPT interruption or cessation.40
All serious adverse events among people taking TPT (SAEs, as defined by the Australian National Health and Medical Research Council).41
Anyone with DILI during treatment or with an abnormal ALT (≥2 × ULN) at baseline will receive an HBV rapid test (if not previously performed). In addition, blood drawn from participants with DILI and matched controls will be stored (with additional consent) for expanded risk factor determination, using an efficient nested case–control study design.
Other adverse events that will be recorded include:
Misclassification or misinterpretation of screening results resulting in mistreatment.
Postpositive screen treatment delay for TB disease (>7 days) and leprosy (>14 days).
Leprosy prophylaxis
All participants who do not commence any other form of treatment are eligible for leprosy prophylaxis using SDR. Those who commence treatment for TB or leprosy, or TPT using 3HP or 3RH, already receive multiple doses of a rifamycin as part of those regimens, which constitutes effective leprosy prophylaxis. Contraindications (box 1) are assessed and if none are found SDR is given as directly observed treatment, dosed according to WHO age and weight bands (with dispersible tablets for children <25 kg).42
Box 1. Contraindications to single-dose rifampicin.
History of liver or kidney disease.
Known pregnancy.
Known allergy to rifampicin.
Signs and/or symptoms of leprosy.
Signs and/or symptoms of TB.
SDR is well tolerated and widely used for PEP in household contacts of people with leprosy43 44 and recognised as an effective measure to reduce leprosy prevalence if applied at a population scale in high incidence settings.20 44 45 Among published studies evaluating leprosy prophylaxis, SDR was reported to be safe with no observed SAEs,43 and we are not aware of any SAEs reported during programmatic implementation. Offering either SDR, 3HP, 3RH, TB treatment and/or leprosy treatment to every participant equates to a single mass drug administration intervention.
Ongoing screening of close contacts of leprosy cases, with PEP provision, will continue beyond the study intervention, supported by the PLF. The PLF have conducted leprosy contact tracing and PEP using SDR in Kiribati since 2010 and are committed to continue this work.
Outcome measures
Primary outcomes
The first primary outcome measure of this study is the difference between TB case notification rates in South Tarawa, recorded from NTP data, during the 12 months before the intervention (2020) and the 12 months after it concludes (2024/5). The denominator is the population of South Tarawa, as reported by the National Statistics Office. This outcome measure has been chosen to reflect the programmatic intent of the intervention, and the aim of the study to effect a step-change in TB prevalence as a pathway to elimination. Annual TB notification rates will be monitored throughout the study period and are expected to first increase as a result of active case finding, before declining towards the end of the intervention and after its completion.
The second primary outcome measure is estimated annual risk of TB infection (ARTI) among primary school children in South Tarawa, measured by TST before and 6 months after the intervention. This outcome measure has been chosen to assess the impact of the intervention on community transmission of TB. Together, the primary outcomes allow us to assess the public health and epidemiological impact of the TB intervention.
Secondary outcomes
Population prevalence of TST positivity in different age bands.
Description of the disease spectrum and risk factors observed among people with TB and/or leprosy.
Comparison of TB case notification rates in South Tarawa (the intervention site) and the rest of Kiribati (control) before, during and after the intervention.
Percentage of participants retained at each step along the ‘cascade of care’46 (invited to screening, TST placed, TST read, completed screening, offered TPT, commenced TPT, completed TPT).
Diagnostic yield of mobile digital CXRs with CAD, compared with sputum testing using Gene Xpert MTB/RIF ultra, in patients who had both tests performed.
Incidence rate of DILI among people taking TPT, and the population attributable fraction of HBV infection and other documented risk factors.
Difference in leprosy case notification rates in South Tarawa, recorded from NLP data, during the 12 months before the intervention (2020) and the 12 months after its conclusion (2024/2025).
Comparison of leprosy case notification rates in South Tarawa (the intervention site) and the rest of Kiribati (control) before, during and after the intervention.
Spatial analysis of TB and leprosy cases detected during the intervention, including data visualisation (mapping and interpolation), identification of spatial clusters, and spatial statistical modelling.
Documenting the cost of the combined intervention, as well as TB and leprosy components separately, to estimate the incremental cost-effectiveness ratio in comparison to modelled outcomes without the intervention
Sample size
For the TB and leprosy case notification rate outcomes, the sample size is the whole population of South Tarawa (total population 65 566 at the 2020 census, Kiribati National Statistics Office 2021). Additional analysis of the sensitivity of our study design to detect an effect on TB and leprosy case notification rates is included as online supplemental files S6 and S7). The ARTI outcome is calculated from estimated TB infection prevalence using the formula ARTI=1 – (1 – TB infection prevalence)1/a, where a=mean age, estimated as 1.8%/year in primary school-aged children (mean age 9 years, TB infection prevalence 15%).47 The ability to detect a 50% reduction in ARTI with a power of 0.8 (alpha of 0.05), comparing two samples of children at baseline and after the intervention, requires a sample size of 2580 children at each measurement (G*Power V.3.1).48 Primary school children will be representatively sampled from the same age categories and schools at both measurements.
Governance
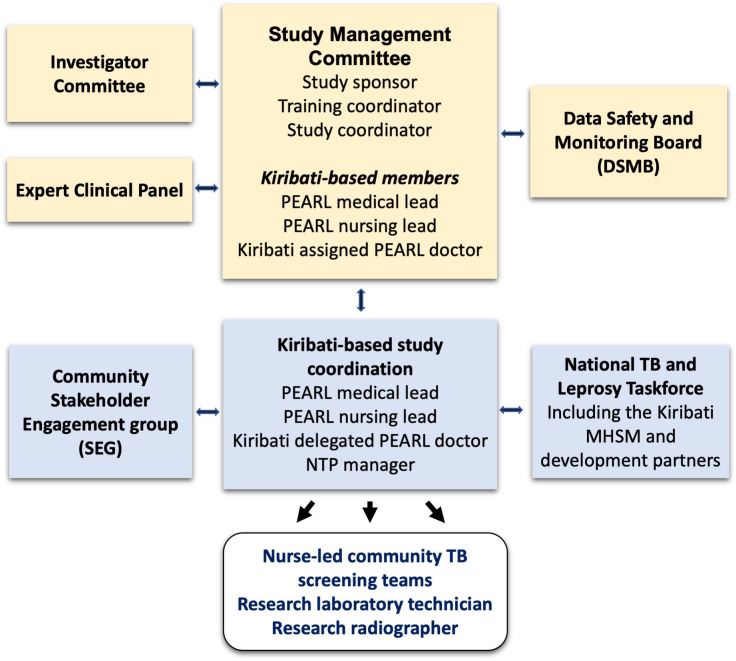
The Study Management Committee (responsible for day-to-day running and monitoring of the study) is composed of the Kiribati-based medical lead, an i-Kiribati doctor nominated by the Kiribati MHSM, the Kiribati-based nursing lead, the study coordinator, the overall study lead and the education coordinator. All SAEs are reported within 1 week of its recognition to the University of Sydney Human Research Ethics Committee and the Kiribati MHMS by the study coordinator. An independent Data Safety and Monitoring Board will assess study progress and the adverse events recorded and reported as described above. Figure 2 provides an overview of governance arrangements.
Figure 2.
Overview of study governance. MHMS, Ministry of Health and Medical Services; NLP, National Leprosy Programme; NTP, National TB programme; PEARL, Pathway to the Elimination of Antibiotic Resistant and Latent tuberculosis in the Pacific.
Ethics and dissemination
Ethical issues
Adults aged ≥18 years provide written informed consent (online supplemental file S8). Written parental or caregiver consent is provided for children and adolescents aged <18 years, with those aged 10–18 years asked to provide verbal assent that is recorded. Participants may voluntarily withdraw from the study at any time. All records are strictly confidential, and all study data will be collected on password-protected electronic devices. A complete database will be stored on a high security data repository administered by the University of Sydney in Sydney, Australia. Relevant data will be extracted for the Kiribati MHMS and NTP as required for clinical or public health purposes, and made available at study completion for future patient care and population benefit.
Particular attention is given to handling of biometric data, which has been included in the study procedures after close consultation with the Kiribati MHMS and extensive collaboration with the biometric identification provider. Biometric identifiers are captured in a standalone mobile application and stored in a secure database maintained by the biometric identification provider, which is separate from the study database and inaccessible to users. Records in the two databases are linked by a unique, randomly generated identifier. When study staff use biometric identification to retrieve a participant record, the linking identifier is accessed by the biometric identification application without further input by the user. Participants are free to refuse or withdraw consent to record biometric identifier data, while still participating in the study. Participants are specifically asked for consent to share biometric identification data with the Kiribati MHMS, and are free to refuse or withdraw.
Dissemination
Study findings will be presented at international conferences and submitted for publication in peer-reviewed journals. Findings will also be disseminated through the websites of collaborating organisations and in writing to donors. Editable resources developed for the study will be posted on the study website for free-to-use access: www.thepearlstudy.org. Future authorship will include all substantial contributors to the work and there will be a statement of the role of the funder and any potential conflicts of interest. There will be due recognition and acknowledgement of study participants, local study staff and contributions made by i-Kiribati colleagues. There is an overarching commitment to involve i-Kiribati colleagues in all aspects of the study design and execution and to invest in local capacity building. Interim and final reports will be shared with the Kiribati MHMS, with regular updates provided to the i-Kiribati public and study participants.
Patient and public involvement
There was no patient involvement in the design of the study. The study is strongly aligned with national priorities in Kiribati, which recognises TB and leprosy as major infectious disease challenges.49 The study was informed by the need for an urgent TB and leprosy control solution in Kiribati, as articulated by the Kiribati MHMS. A community Stakeholder Engagement Group comprised of i-Kiribati TB and leprosy survivors, local lay leadership and health practitioners will provide community feedback and guidance throughout the intervention period. Patient advisors will be thanked in the acknowledgements of future study publications. Testing TB elimination strategies in the Pacific is also aligned with the ‘Regional Framework for Action on Implementation of the End TB Strategy in the Western Pacific.50 The framework calls for a paradigm shift in TB control and articulates a need to ‘integrate diagnosis and management of LTBI into systematic screening for TB disease among high-risk populations’.50 51
Training, monitoring and evaluation
Building skills, knowledge and workforce capacity is seen as an essential component of the study. Regular staff development training will be conducted by the Australian Respiratory Council. Senior research staff and the Kiribati MHMS will conduct ongoing internal monitoring. Data quality control and critical review of processes will be performed on a weekly basis throughout the study by the data manager. Protocol compliance, recruitment, screening and treatment practice, and laboratory processes will be externally reviewed on a quarterly basis, with physical inspection whenever possible.
Data management and analysis
Data will be collected offline in a standardised fashion and captured on electronic tablets, with daily upload into a secure web-based REDcap database.39 Participants will be assigned a unique study identifier at enrolment, which will be matched with identifying data including a photograph and a biometric identifier (Simprints, Cambridge, UK). This will add accuracy to retrieval of patient records and reduce misidentification. The data manager will review data uploads on a daily basis. At the end of the intervention and while awaiting conduct of the final TST survey among school children, we will complete data cleaning and start analysis. At this time, we will also consolidate the transfer of knowledge and skills and focus on assisting the Kiribati MHMS with longer term planning. A detailed data analysis plan will be drafted focusing on the primary and secondary outcome measures articulated above. In order to assess the durability of the effect we will track annual TB and leprosy case notification numbers beyond the end of the project, which should also inform future interventions and complementary studies.
Discussion
Ambitious action is needed to change the course of the global TB epidemic, and to make up ground lost to the COVID-19 pandemic. Scaling up access to systematic screening and TPT for high-burden communities has been identified as a key intervention to achieve this,52 and is reflected in multilateral targets and commitments. For example, at the UN High Level Meeting on TB held in 2018, countries committed to providing 20 million courses of TPT to HIV-negative adult household contacts. Less than 1% of this target has been met, despite abundant evidence that these individuals are at high risk of developing TB and perpetuating the cycle of transmission at the community level.
Recently published WHO guidelines on systematic screening for TB recommend that population level systematic screening can be adopted where the estimated incidence is above 500/100 000—this threshold is met in South Tarawa. Current WHO TPT guidelines state that population-based TB infection testing and treatment is not considered feasible in the absence of locally available tests for TB infection and reliable tests to rule out TB disease, and while it is recognised that it may assist TB elimination efforts, the public health benefit remains unproven.32 A population-wide intervention to diagnose and treat TB disease and infection was recently implemented in the Marshall Islands; the ‘TB and Leprosy Free Majuro’53 project demonstrated the safety and feasibility of this approach in a Pacific context. For leprosy, WHO acknowledges that universal leprosy prophylaxis, in addition to active case finding, can be valuable to assist elimination efforts,43 which presents a major opportunity for combined TB and leprosy elimination efforts.
Implementing a comprehensive TB elimination strategy in South Tarawa has important benefits for TB control. First, population-wide active case finding will facilitate early TB disease detection and treatment, reducing associated morbidity and mortality and limiting ongoing TB transmission within the community. Second, detection and treatment of TB infection will reduce future disease reactivation and greatly increase the durability of the positive impact achieved by the intervention. The feasibility of such an ambitious project is highly dependent on support from the people of South Tarawa, strong political commitment from the Kiribati government and cooperation with relevant partners and stakeholders. Third, this study will provide detailed information on the prevalence of TB infection, active TB and leprosy, and document the impact of population-wide screening approaches to inform modelling of TB and leprosy control strategies in the Pacific.9 20
Beyond the domain of TB control, this study will also strengthen the health system of Kiribati. Updated household-level and individual-level information collected during the study will facilitate public health interventions in other disease areas as well. Leprosy screening is included in the main intervention, and a proportion of participants will be screened for HBV; these activities will be accompanied by system changes that align the efforts of the three disease control programmes and could serve as an example in other areas. Strengthened laboratory and radiology capacity will have benefits for the health system overall, for example, expansion of access to genotypic diagnosis and digital X-ray systems with CAD software would both be of direct benefit in the event of a COVID-19 outbreak. Ultimately, this study is aligned with national and regional priorities for health system strengthening and universal healthcare.
Study limitations include the reliance on case notification rates as a primary outcome measure, which reflects the operational nature of the intervention. Early diagnosis and treatment of cases through active case finding using sensitive tests may increase the measured effect, due to lead time bias. The provision of TPT to all participants with TB infection will reduce the number of incipient disease cases; also adding to potential lead time bias. However, the impact measured will reflect ‘real world’ reductions achieved and monitoring TB incidence rates before, throughout, and for an extended period after the intervention will allow us to reflect on the potential impact of lead time bias and to assess the durability of the reductions achieved. Comparing ARTI before and after the intervention provides an objective marker of community transmission, which is not affected by lead time bias. This could also be supplemented by a third ARTI assessment 3–5 years after study completion to assess the durability of the effect, if resources are available.
The PEARL study benefits from extensive collaboration with the Kiribati MHMS, NTP, NLP and other agencies, as well as established partnerships with the PLF, the WHO Western Pacific Regional Office (Manila, Philippines), the office of the WHO Representative in the South Pacific (Suva, Fiji), the United Nations Development Programme and the Australian Department of Foreign Affairs and Trade among others. In addition, extensive community engagement, communication and mobilisation forms the cornerstone of study implementation. Pacific island countries and territories like Kiribati are in a unique position, given their geographic isolation and limited population size, to ‘lead the way’ by implementing ambitious elimination strategies that serve as proof-of-principle for others to learn from and replicate.
The PEARL study addresses the need for rigorous implementation science to assess the feasibility and impact of population-wide active case finding and treatment, combined with testing and treatment for TB infection, to durably reduce the TB burden in high incidence settings. The study also explores complementarity between TB and leprosy elimination efforts, with the promise of developing scalable strategies suitable for remote settings with high disease burdens.
Supplementary Material
Acknowledgments
The authors would like to extend sincere thanks to Jill Tomlinson, the CEO of the Pacific Leprosy Foundation and to the staff of the Kiribati NTP and NLP for their outstanding support and contributions to the PEARL study.
Footnotes
Contributors: BJM wrote the initial study proposal. MC and JH contributed equally to this paper. MC, JH, GJF, ET, AT, BE, TI, JMT, STC, AC, GBM, WJB and BJM made important intellectual contributions to the final study protocol. GBM and GJF performed sample size calculations. MC prepared the first draft of the manuscript. The funding agency played no part in any aspect of the study, nor the decision to submit this manuscript for publication.
Funding: This work is supported by the Australian Medical Research Future Fund (MRFF) grant, APP1200755 and the Pacific Leprosy Foundation. The investigators are also supported by the National Health and Medical Research Council Centre of Research Excellence in Tuberculosis Control on both sides of the border (APP1153493). The Australian Department of Foreign Affairs and Trade (DFAT) awarded additional equipment funding to the project.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.WHO . Global tuberculosis report 2021. Geneva: Organisation WH, 2021. [Google Scholar]
- 2.Chambers ST, Ioteba N, Timeon E, et al. Surveillance of leprosy in Kiribati, 1935-2017. Emerg Infect Dis 2020;26:833–40. 10.3201/eid2605.181746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO . Political Declaration of the un General-Assembly high-level meeting on the fight against tuberculosis. In: World Health Organisation WTP, 2019. https://www.who.int/publications/m/item/political-declaration-of-the-un-general-assembly-high-level-meeting-on-the-fight-against-tuberculosis [Google Scholar]
- 4.WHO . The end TB strategy, 2014. Available: https://www.who.int/tb/strategy/End_TB_Strategy.pdf
- 5.MSF . Step up for TB report 2020. partnership TST, 2020. https://www.msf.org/step-tb-report-2020 [Google Scholar]
- 6.Comstock GW, Ferebee SH, Hammes LM. A controlled trial of community-wide isoniazid prophylaxis in Alaska. Am Rev Respir Dis 1967;95:935–43. 10.1164/arrd.1967.95.6.935 [DOI] [PubMed] [Google Scholar]
- 7.Marks GB, Nguyen NV, Nguyen PTB, et al. Community-Wide screening for tuberculosis in a high-prevalence setting. N Engl J Med 2019;381:1347–57. 10.1056/NEJMoa1902129 [DOI] [PubMed] [Google Scholar]
- 8.Dye C, Glaziou P, Floyd K, et al. Prospects for tuberculosis elimination. Annu Rev Public Health 2013;34:271–86. 10.1146/annurev-publhealth-031912-114431 [DOI] [PubMed] [Google Scholar]
- 9.Ragonnet R, Williams BM, Largen A. Estimating the long-term effects of population-wide screening for latent and active tuberculosis in the Republic of the Marshall islands. medRxiv 2021. 10.1101/2021.03.03.21252859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Churchyard GJ, Swindells S. Controlling latent TB tuberculosis infection in high-burden countries: a neglected strategy to end TB. PLoS Med 2019;16:e1002787. 10.1371/journal.pmed.1002787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Getahun H, Matteelli A, Chaisson RE, et al. Latent Mycobacterium tuberculosis infection. N Engl J Med 2015;372:2127–35. 10.1056/NEJMra1405427 [DOI] [PubMed] [Google Scholar]
- 12.Houben RMGJ, Dodd PJ. The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLoS Med 2016;13:e1002152. 10.1371/journal.pmed.1002152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saunders MJ, Evans CA. Ending tuberculosis through prevention. N Engl J Med 2019;380:1073–4. 10.1056/NEJMe1901656 [DOI] [PubMed] [Google Scholar]
- 14.Rangaka MX, Cavalcante SC, Marais BJ, et al. Controlling the seedbeds of tuberculosis: diagnosis and treatment of tuberculosis infection. Lancet 2015;386:2344–53. 10.1016/S0140-6736(15)00323-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO . WHO operational handbook on tuberculosis: module 1: prevention: tuberculosis preventive treatment, 2020. [Google Scholar]
- 16.Group WB . Kiribati health financing system assessment: spend Bette. Washington, DC: Bank W, 2018. [Google Scholar]
- 17.Organization WH . The end TB strategy. World Health Organization, 2015. [Google Scholar]
- 18.Aleksic E, Merker M, Cox H, et al. First molecular epidemiology study of Mycobacterium tuberculosis in Kiribati. PLoS One 2013;8:e55423. 10.1371/journal.pone.0055423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morishita FVK, Lowbridge C, Rahevar K, et al. Epidemiology of tuberculosis in the Western Pacific region: progress toward the 2020 milestone of the end TB strategy. WPSAR. In Press 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilkison C, Chambers S, Blok DJ, et al. Predicting the impact of household contact and mass chemoprophylaxis on future new leprosy cases in South Tarawa, Kiribati: a modelling study. PLoS Negl Trop Dis 2019;13:e0007646. 10.1371/journal.pntd.0007646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bank TW. Data catalog: GDP per capita. Bank TW, 2019. [Google Scholar]
- 22.Bank TW . Incidence of tuberculosis (per 100,000 people). World Health organization, global tuberculosis report 2020, 2021. Available: https://data.worldbank.org/indicator/SH.TBS.INCD?most_recent_value_desc=true:TheWorldBank
- 23.Organisation WH . Leprosy - New cases detection rate per 100 000 population. The Global Health Observatory, 2020. World health data platform. Available: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/leprosy-new-cases-detection-rate-per-100-000-population
- 24.Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract 2019;157:107843. 10.1016/j.diabres.2019.107843 [DOI] [PubMed] [Google Scholar]
- 25.Hoy D, Kienene T, Reiher B, et al. Battling tuberculosis in an island context with a high burden of communicable and non-communicable diseases: epidemiology, progress, and lessons learned in Kiribati, 2000 to 2012. Int J Infect Dis 2015;30:135–41. 10.1016/j.ijid.2014.11.025 [DOI] [PubMed] [Google Scholar]
- 26.WHO . WHO rapid communication: molecular assays as initial tests for the diagnosis of tuberculosis and rifampicin resistance, 2020. https://www.who.int/tb/publications/2020/rapid-communications-molecular-assays/en/ [Google Scholar]
- 27.Organisation WH . WHO consolidated guidelines on tuberculosis module 2: screening – systematic screening for tuberculosis diseas. global tuberculosis programme, 2021. [PubMed] [Google Scholar]
- 28.Madhani F, Maniar RA, Burfat A, et al. Automated chest radiography and mass systematic screening for tuberculosis. Int J Tuberc Lung Dis 2020;24:665–73. 10.5588/ijtld.19.0501 [DOI] [PubMed] [Google Scholar]
- 29.Patel MK, Wannemuehler K, Tairi R, et al. Progress towards achieving hepatitis B control in the Cook Islands, Niue, Tokelau, and Kiribati. Vaccine 2016;34:4298–303. 10.1016/j.vaccine.2016.06.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jackson K, Tekoaua R, Holgate T, et al. Hepatitis B and D in the Pacific islands of Kiribati. J Clin Virol 2020;129:104527. 10.1016/j.jcv.2020.104527 [DOI] [PubMed] [Google Scholar]
- 31.Fox GJ, Oxlade O, Menzies D. Fluoroquinolone therapy for the prevention of multidrug-resistant tuberculosis in contacts. A cost-effectiveness analysis. Am J Respir Crit Care Med 2015;192:229–37. 10.1164/rccm.201501-0069OC [DOI] [PubMed] [Google Scholar]
- 32.WHO . WHO consolidated guidelines on tuberculosis: tuberculosis preventive treatment: module 1: prevention: tuberculosis preventive treatment, 2020. [PubMed] [Google Scholar]
- 33.Walker RE, Bass S, Srinivas P, et al. Evaluation of 3 months of once-weekly rifapentine and isoniazid for latent tuberculosis infection. Ann Pharmacother 2020;54:457–63. 10.1177/1060028019888855 [DOI] [PubMed] [Google Scholar]
- 34.Alvarez GG, Van Dyk D, Mallick R, et al. The implementation of rifapentine and isoniazid (3Hp) in two remote Arctic communities with a predominantly Inuit population, the Taima TB 3Hp study. Int J Circumpolar Health 2020;79:1758501. 10.1080/22423982.2020.1758501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wheeler C, Mohle-Boetani J, Rates C. Completion rates, adverse effects, and costs of a 3-month and 9-month treatment regimen for latent tuberculosis infection in California inmates, 2011-2014. Public Health Rep 2019;134:71S–9. 10.1177/0033354919826557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamada Y, Ford N, Schenkel K, et al. Three-Month weekly rifapentine plus isoniazid for tuberculosis preventive treatment: a systematic review. Int J Tuberc Lung Dis 2018;22:1422–8. 10.5588/ijtld.18.0168 [DOI] [PubMed] [Google Scholar]
- 37.Pease C, Hutton B, Yazdi F, et al. A systematic review of adverse events of rifapentine and isoniazid compared to other treatments for latent tuberculosis infection. Pharmacoepidemiol Drug Saf 2018;27:557–66. 10.1002/pds.4423 [DOI] [PubMed] [Google Scholar]
- 38.Sterling TR, Villarino ME, Borisov AS, et al. Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med 2011;365:2155–66. 10.1056/NEJMoa1104875 [DOI] [PubMed] [Google Scholar]
- 39.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Health N, Council MR. Guidance: safety monitoring and reporting in clinical trials involving therapeutic goods, 2016. [Google Scholar]
- 41.NHMRC . Guidance: safety monitoring and reporting in clinical trials involving therapeutic goods, 2016. Available: https://www.nhmrc.gov.au/about-us/publications/safety-monitoring-and-reporting-clinical-trials-involving-therapeutic-goods:NationalHealthandMedicalResearchCouncil
- 42.WHO . Guidelines for the diagnosis, treatment and prevention of leprosy, 2018. https://apps.who.int/iris/handle/10665/274127 [Google Scholar]
- 43.Richardus JH, Tiwari A, Barth-Jaeggi T, et al. Leprosy post-exposure prophylaxis with single-dose rifampicin (LPEP): an international feasibility programme. Lancet Glob Health 2021;9:e81–90. 10.1016/S2214-109X(20)30396-X [DOI] [PubMed] [Google Scholar]
- 44.Schoenmakers A, Mieras L, Budiawan T, et al. The state of affairs in post-exposure leprosy prevention: a descriptive meta-analysis on immuno- and Chemo-Prophylaxis. Res Rep Trop Med 2020;11:97–117. 10.2147/RRTM.S190300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tiwari A, Dandel S, Djupuri R, et al. Population-Wide administration of single dose rifampicin for leprosy prevention in isolated communities: a three year follow-up feasibility study in Indonesia. BMC Infect Dis 2018;18:324. 10.1186/s12879-018-3233-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alsdurf H, Hill PC, Matteelli A, et al. The cascade of care in diagnosis and treatment of latent tuberculosis infection: a systematic review and meta-analysis. Lancet Infect Dis 2016;16:1269–78. 10.1016/S1473-3099(16)30216-X [DOI] [PubMed] [Google Scholar]
- 47.Shanaube K, Sismanidis C, Ayles H, et al. Annual risk of tuberculous infection using different methods in communities with a high prevalence of TB and HIV in Zambia and South Africa. PLoS One 2009;4:e7749. 10.1371/journal.pone.0007749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Faul F, Erdfelder E, Buchner A, et al. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods 2009;41:1149–60. 10.3758/BRM.41.4.1149 [DOI] [PubMed] [Google Scholar]
- 49.Office DoNEP . Kiribati development plan 2016-19, 2016. Government of Kiribati. Available: www.kiribati.gov.ki
- 50.WHO . Regional framework for action on implementation of the end TB strategy in the Western Pacific, 2016. Available: http://iris.wpro.who.int/handle/10665.1/13131
- 51.Viney KLC, Morishita F, Rahevar K. A critical evaluation of the ‘Regional Framework for Action on Implementation of the End TB Strategy in the Western Pacific Region, 2016-2020. WHO Bull. In press 2020. [Google Scholar]
- 52.Mohr-Holland E, Douglas-Jones B, Apolisi I, et al. Tuberculosis preventive therapy for children and adolescents: an emergency response to the COVID-19 pandemic. Lancet Child Adolesc Health 2021;5:159–61. 10.1016/S2352-4642(21)00003-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brostrom R, Largen A, Konelios-Langinlur M, et al. OA-14-409-01 voyage to TB elimination: a mass TB treatment and prevention campaign in the Marshall Islands. 50th world conference on lung health of the International Union against tuberculosis and lung disease (the Union). Hyderabad: International Union Against Tuberculosis and Lung Disease, 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-055295supp001.pdf (9.3MB, pdf)