Abstract
Background
Excessive daytime sleepiness is a common symptom of myotonic dystrophy. Psychostimulants are drugs increasingly used to treat hypersomnia in myotonic dystrophy.
Objectives
To search systematically for, and combine all evidence from, randomized trials relating to the effects of psychostimulants in myotonic dystrophy patients with hypersomnia.
Search methods
We searched the Cochrane Neuromuscular Disease Specialized Register (October 2010), the Cochrane Central Register of Controlled Trials (CENTRAL) (19 October 2010, issue 4, 2010 in the Cochrane Library), MEDLINE (January 1966 to October 2010) and EMBASE (January 1980 to October 2010) for randomized trials concerning psychostimulants in myotonic dystrophy, checked the bibliographies of identified papers and made enquiries of the authors of the papers.
Selection criteria
We considered all randomized or quasi‐randomized trials that have evaluated any type of psychostimulant (versus a placebo or no treatment) in children or adults with proven myotonic dystrophy and hypersomnia.
Data collection and analysis
Potentially relevant papers were scrutinized by two authors and the selection of eligible studies was agreed by them and a third author. Data were extracted by one author and checked by a second author.
Main results
Primary outcome Mean improvement in the maintenance of wakefulness test was available for two of the five identified trials accounting for 48 participants. The mean difference +2.52(95% confidence interval (CI) ‐2.32 to +7.37), was not significant and there was marked heterogeneity across these studies (I2= 50%).
Secondary outcomes Mean improvement in the Epworth Sleepiness Scale was available in four trials accounting for 101 participants. The mean difference was ‐2.26 (95% CI ‐3.78to ‐0.73), significantly in favor of modafinil with marked heterogeneity across the studies (I2= 84%). There was no evidence for any treatment benefit on the multiple sleep latency test or quality of life.
Authors' conclusions
There is low quality evidence from two small trials that psychostimulants do not significantly improve the maintenance of wakefulness test in myotonic dystrophy. There is low quality evidence from four studies that modafinil significantly improves the Epworth Sleepiness Scale. More randomized trials are needed to evaluate the efficacy and safety of psychostimulants.
Plain language summary
Drugs that increase alertness (psychostimulants) for excessive daytime sleepiness (hypersomnia) in myotonic dystrophy
Myotonic dystrophy is an inherited muscular dystrophy causing muscle weakness and wasting. Many people with myotonic dystrophy complain about excessive daytime sleepiness. This symptom is related to disordered central respiratory control. Psychostimulants are drugs that increase alertness and include caffeine, amphetamine, selegiline, methylphenidate and modafinil. In this updated review there were few randomized controlled trials which evaluated the efficacy and safety of psychostimulants in myotonic dystrophy. One randomized controlled trial of selegiline involving 11 participants did not demonstrate any benefit. Four studies of another drug modafinil suggested inconsistent and slight benefits. Only two of these studies used the gold standard test, a sleepiness scale, to evaluate hypersomnia and found non significant improvement. In these four studies modafinil seemed well tolerated. Further randomized trials are needed to determine the utility of psychostimulants for myotonic dystrophy.
Summary of findings
Summary of findings for the main comparison. Psychostimulants for hypersomnia (excessive daytime sleepiness) in myotonic dystrophy.
| Psychostimulants for hypersomnia (excessive daytime sleepiness) in myotonic dystrophy | ||||||
| Patient or population: patients with hypersomnia (excessive daytime sleepiness) in myotonic dystrophy Settings: outpatients Intervention: Psychostimulants | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Psychostimulants | |||||
| improvement in the maintenance of wakefulness test Follow‐up: mean 4 weeks | The mean improvement in the maintenance of wakefulness test in the intervention groups was 2.11 higher (1.98 lower to 6.21 higher) | 48 (2 studies) | ⊕⊕⊝⊝ low1,2 | |||
| improvement in the Epworth Sleepiness Score Follow‐up: mean 4 weeks | The mean improvement in the Epworth Sleepiness Score in the intervention groups was 2.26 lower (3.78 to 0.73 lower) | 101 (4 studies3) | ⊕⊕⊝⊝ low | |||
| improvement in multiple sleep latency test Follow‐up: mean 4 weeks | The mean improvement in multiple sleep latency test in the intervention groups was 1.82 lower (5.57 lower to 1.93 higher) | 48 (2 studies4) | ⊕⊕⊝⊝ low | |||
| quality of life scale ‐ mean improvement in total score of Short Form 36 Follow‐up: mean 4 weeks | The mean quality of life scale ‐ mean improvement in total score of Short Form 36 in the intervention groups was 1.27 higher (3.63 lower to 6.17 higher) | 48 (2 studies4) | ⊕⊕⊝⊝ low | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 There was some heterogeneity in the results that we could not explain 2 Studies had a small sample size 3 3 studies had a crossover design: Talbot 2003 (n=20), MacDonald 2002 (n=40), and Wintzen, 2007 (n=13) 4 Study by Talbot 2003 had a crossover design, n=20
Background
Myotonic dystrophy is the most common form of adult muscular dystrophy, with a prevalence of one in 8000 (Harper 1979). This multisystem disorder is dominantly inherited and caused by amplification of an unstable trinucleotide (CTG)n repeat (Brook 1992; Buxton 1992; Fu 1992; Harley 1992; Mahadevan 1992) in a transcript encoding a serine/threonine kinase, the dystrophia myotonica‐protein kinase (Dunne 1994). The mechanisms by which the expanded trinucleotide repeat causes a dominant biochemical defect and the varied clinical phenotype are unknown.
Excessive daytime somnolence or hypersomnia has long been recognized as a prominent symptom of myotonic dystrophy (Gillam 1964; Harper 1979), and has been documented in 40 to 50% of people with the disease (Manni 1991; Rubinsztein 1998). The mechanisms of somnolence in myotonic dystrophy are not fully understood. However, it is clear from several studies that hypersomnia is not related to the degree of muscular disability or the degree of hypercapnia that might result from associated respiratory weakness (Bégin 1997; Gillam 1964; Guilleminault 1978; Manni 1991). In addition, the daytime somnolence is not related to the disturbance of sleep pattern or night time breathing abnormalities which are common in myotonic dystrophy (Gilmartin 1991; Manni 1991; Van der Meché 1994). Loss of serotonin‐containing neurons in the median raphe of people with myotonic dystrophy who had daytime somnolence argues strongly in favor of a central origin for the hypersomnia (Ono 1998).
In an observational study, the central stimulant methylphenidate produced sustained (up to two to six years) improvement in daytime sleepiness in 7 out of 11 participants with myotonic dystrophy (Van der Meché 1994). Another open‐label trial conducted in 11 participants with myotonic dystrophy showed that prolonged treatment (16.4 weeks on average) with 200 to 400 mg of modafinil significantly improved daytime sleepiness (Damian 2001).
This systematic review was designed to determine the extent and quality of the evidence for the role of psychostimulants in the management of hypersomnia in myotonic dystrophy.
Objectives
The primary aim of the present systematic review was to search systematically for, and combine all evidence from, randomized trials relating to the effects of psychostimulants in myotonic dystrophy patients with hypersomnia.
Methods
Criteria for considering studies for this review
Types of studies
All randomized or quasi‐randomized trials with or without blinding were included.
Types of participants
Children and adults who met the established clinical and electromyographic criteria for myotonic dystrophy (Griggs 1989) were considered.
Types of interventions
All psychostimulants, including caffeine, amphetamine, methylphenidate, selegiline, modafinil, were considered.
Types of outcome measures
Primary outcomes
Mean improvement in the maintenance of wakefulness test. The maintenance of wakefulness test is a standardized tool to evaluate hypersomnia and consists usually of five polygraphic recordings at two‐hour intervals made on the day after an overnight polysomnography. During each recording the patient is in the sitting position and is asked to stay awake, and sleep latencies are calculated from the four first recordings (Mitler 1982). Results are expressed in minutes with higher scores representing reduced hypersomnia.
Secondary outcomes
Mean improvement in a sleepiness scale, such as the Epworth Sleepiness Scale (Johns 1991).
Mean improvement in the multiple sleep latency test. The multiple sleep latency test is a standardized tool to evaluate hypersomnia and consists usually of four polygraphic recordings at two hour intervals made on the day after an overnight polysomnography. During each recording the patient is lying in the recumbent position, asked to sleep and is allowed 20 minutes to fall asleep, and sleep latencies are calculated (Carskadon 1986). The results are expressed in minutes with lower scores representing hypersomnia.
Ambulatory 24 hour sleep‐wake monitoring.
Sleep studies, i.e. apnea‐hypopnea index or mean oxygen saturation or the time spent with an arterial oxygen saturation below 90%.
Quality of life, as assessed for example by the SF36, for which we computed a total score by summing the mental and physical component.
Adverse events.
The primary and the secondary outcomes were recorded separately after two to four weeks (short‐term effects) and after six to 12 months (long‐term effects) following treatment initiation.
Main outcomes for 'Summary of findings' table
The main outcomes to be included in the 'Summary of findings' tables included the maintenance of wakefulness test, the Epworth Sleepiness Scale, the multiple sleep latency test and quality of life.
Search methods for identification of studies
We searched the Cochrane Neuromuscular Disease Group Specialized Register with 'myotonic dystrophy' OR 'Steinert disease' OR 'dystrophia myotonica' OR 'myotonia atrophica' AND 'hypersomnia' OR 'somnolence' OR 'sleepiness' AND 'central stimulants' OR 'psychostimulants' OR 'caffeine' OR 'amphetamine' OR 'methylphenidate' OR 'selegiline' OR 'modafinil' as search terms.
All references in the identified trial were checked. Contact with the authors of the only trial we found in the original version of the review did not identify any additional published or unpublished data.
Electronic search strategies
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (19 October 2010, Issue 4, 2010 in the Cochrane Library), MEDLINE (January 1966 to October 2010), and EMBASE (January 1980 to October 2010). The strategies are given in Appendix 1, Appendix 2 and Appendix 3.
Data collection and analysis
Selection of studies
Two authors independently checked titles and abstracts identified from the database. Two authors obtained the full text of all studies of possible relevance for independent assessment. The authors decided which trials fitted the inclusion criteria, and graded their methodological quality. The authors resolved any disagreement by discussion between the authors. We contacted authors of trials for clarification where necessary.
Data extraction and management
Two authors performed data extraction independently with specific data extraction forms and we contacted the authors of trials systematically to provide missing data where possible. One author (DA) entered data into the computer and the others checked the data entry.
Assessment of risk of bias in included studies
Two authors assessed the risk of bias in the included trials independently, with particular emphasis on appropriateness of the sequence generation (YES = a random component in the sequence generation is described, NO = a non‐random component is described, UNCLEAR = insufficient information is available), on appropriateness of treatment allocation concealment (YES = method used avoids that investigators foresee assignment; NO = Investigators could possibly foresee assignment; UNCLEAR = insufficient information is available). We also assessed the adequacy of blinding of participants, personnel and outcome assessors, and of addressing the issue of incomplete data assessment, and selective outcome reporting. Finally, we also checked other potential problems that may have resulted in a risk of bias, i.e. protocol violations, early stopping of the trial, extreme baseline imbalance, any other problem in the design or the conduct of the trial that was reported by the authors. Where there was uncertainty, we contacted authors of trials for clarification. We resolved any disagreement by discussion.
Measures of treatment effect
For each outcome measure 2 x 2 tables, summarizing the number of people who experienced the event or outcome in each comparison group and the total number in each group, were computed. These tables were organized so that a beneficial effect of treatment was associated with a risk ratio (RR) < 1 or a risk difference (RD) < 0. Intention‐to‐treat analyses were performed. Statistical calculations were performed using Review Manager (RevMan) 5 when possible.
Assessment of reporting biases
If sufficient studies had been discovered, evidence of publication bias was to be investigated using the funnel plot method.
Data synthesis
A weighted treatment effect was to be calculated across trials. The results were to be expressed as risk ratios (RR) with 95% confidence intervals (CI), numbers needed to treat (NNT) for dichotomous outcomes and weighted mean differences (WMD and 95% CI) for continuous outcomes. When cross‐over trials were included in the analysis, treatment effects were summarized as mean difference between treatment effects and standard error, and analyzed by general inverse variance (GIV). Methods based on a random‐effects model were to be considered only in case of heterogeneity. If significant heterogeneity was found, sensitivity analysis was to be performed to identify the trials and the factors responsible (related to patient selection or treatment).
Subgroup analysis and investigation of heterogeneity
Subgroup analysis was to be performed considering sleep‐apnea factors, obesity (defined by a body mass index above 28 kg/m2) and upper airway obstruction (i.e. nasal obstruction, long or hypertrophic soft palate, small jaw, retroposition of the jaw).
Results
Description of studies
We first searched in May 2001 and found only one randomized controlled trial (Antonini 1997). An update in January 2006 found two new potentially relevant studies (MacDonald 2002; Talbot 2003).
For the 2010 update, the number of papers found by the current strategies were: MEDLINE = 15 (8 new papers), EMBASE = 19 (7 new papers), NMD REGISTER = 6 (3 new papers), CENTRAL = 9. From these papers, for the 2010 update, we found two new relevant studies (Orlikowski 2009; Wintzen 2007), making a total of five relevant randomized trials. Four trials had a cross‐over design and one was conducted on parallel groups (Orlikowski 2009). One study (Antonini 1997) had 11 participants, but one dropped out because of poor compliance. Five participants were allocated to receive placebo for 30 days and then, after a 30‐day washout period they received 20 mg daily of selegiline for another 30‐day period. The remaining five participants were randomized to receive selegiline first and then after a 30‐day washout period, placebo. The remaining four trials have investigated the efficacy and safety of modafinil (MacDonald 2002; Orlikowski 2009; Talbot 2003; Wintzen 2007).
One study included 40 adults with myotonic dystrophy who were allocated to receive modafinil (or a placebo) at a dose of 100 mg every twelve hours for one week and then 100 mg in the morning and 200 mg in the evening for one week (MacDonald 2002). The primary efficacy variable in this study was the Epworth Sleepiness Scale. The authors used a modified version of the Epworth Sleepiness Scale from a 4‐point ordinal scale to a 10‐cm visual analogue scale, with the four descriptors (no chance of dozing, slight chance of dozing, moderate chance of dozing, high chance of dozing) centred at 0.5, 3.5, 6.5, and 9.5 cm. Patients made a single mark across the scale at the point corresponding to their perceived chance of falling asleep. Secondary endpoints were the Stanford Sleepiness Scale 17 (SSS), the vigour‐activity and fatigue‐inertia factors of the Profile of Mood States and the energy–fatigue scale from the RAND 36‐Item Health Survey. Modafinil reduced the Epworth Sleepiness Scale score (248 mm on average, 95% CI 220 to 276 versus 309 mm, 95% CI 281 to 336 mm; P < 0.001), and the Standford Sleepiness Scale score (3.05, 95% CI 2.77 to 3.33 versus 3.45, 95% CI 3.45 to 3.71; P < 0.05). On the Profile of Mood State, modafinil decreased the fatigue‐inertia score (P < 0.001) and increased the vigour‐activity and tension‐activity score (P < 0.001), with a decrease in the total mood disturbance score (P < 0.05). On the RAND 36‐Item Health Survey score, modafinil enhanced both quality of life measures of energy (P < 0.001) and health change (P < 0.05). In this study, 30 participants reported a total of 83 adverse events (65 on modafinil and 18 on placebo), mostly during the first week of treatment. Adverse events that were reported more than once included headache (n = 15), anorexia (n = 6), nausea (n = 6), insomnia (n = 5), anxiety (n = 4), dry mouth (n = 4), dyspepsia (n = 4), dizziness (n = 3), nervousness (n = 3), and tachycardia (n = 2). Four randomized participants withdrew, one during the placebo phase in the first week for a reason which was not drug related, and three withdrew due to adverse events, one in the placebo phase and two in the treatment phase.
Another study (Talbot 2003) was conducted in 20 adults with myotonic dystrophy and an Epworth Sleepiness Scale score of 10 or more. The participants were allocated to modafinil or a placebo for two periods of four weeks each separated by a two‐week washout period. Modafinil was given orally as a single morning dose of 100 mg for the first five days and of 200 mg for the remaining days (i.e. study days 6 to 28). This study had two primary outcome measures, the Epworth Sleepiness Scale and a modified maintenance of wakefulness test. This test involved the participants resisting sleep while lying semi‐recumbent in a darkened room for a maximum of 40 minutes on three or four occasions during the day. The participants were asked to tap a proximity detector with a finger in response to a dim red light‐emitting‐diode flashing regularly every three seconds. Sleep was defined as a failure to respond for 21 seconds (or seven responses), and was not based upon electroencephalographic recording. This showed that modafinil reduced the Epworth Sleepiness Scale score slightly but not significantly. Medians varied from 14 to 11 with modafinil versus 13 to 14 with placebo, P = 0.185). At four weeks of treatment the Epworth Sleepiness Scale score was lower in the treated group than in the placebo group (medians, 11 versus 14, P = 0.049). The changes in the modified maintenance of wakefulness score were not significantly different between modafinil and placebo. Medians varied from 31.7 to 40 with modafinil and from 33.3 to 28.7 with placebo (P = 0.063). However, after four weeks of treatment the median score was higher in the modafinil arm compared to placebo arm (40 versus 28.7, P = 0.006). There was no significant difference between modafinil and placebo for secondary outcomes that included a driving stimulation test and the SF‐36 quality of life scale. In this study, no participant withdrew due to adverse events, and one participant withdrew before the final visit for a reason which was not drug related.
A fourth study included 13 patients (Wintzen 2007). Treatments were given for two periods of two weeks, separated by a one‐week washout period. Modafinil was given at the dose of 200 mg per day for the first week and the patients could double the dose during the second week. The primary outcome was the increase in spontaneous activity based on a structured interview of both the patient and the closest relative. The score based on the interview ranged from 0 (no improvement) to five (substantial improvement). Additional outcomes included the RAND‐36 questionnaire, and the Epworth Sleepiness Scale. There was no evidence for any difference between treatment arms, except for the Epworth Sleepiness Scale (‐3.7 versus 0.2, P = 0.015).
In the last study (Orlikowski 2009), 28 patients with genetically proven myotonic dystrophy and an Epworth Sleepiness Scale of more than 10 and onset of sleep in multiple sleep latency test in 8 minutes or less were randomized to receive either modafinil or placebo at a dose of 300 mg per day for 4 weeks. The primary outcome was maintenance of wakefulness test. At four weeks, mean (SD) maintenance of wakefulness test was 15.8 (3.8) in the placebo group and 16.4 (3.3) in the modafinil group (P = 0.93). There was no evidence for a difference between the two groups for any secondary outcome. The differences in baseline and 4 week Epworth Sleepiness Scale values were derived from data provided by the study statistician.
Risk of bias in included studies
All five studies had low risk of bias (Figure 1).
1.
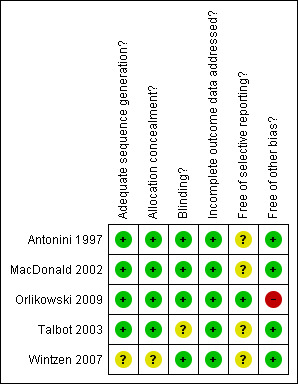
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Effects of interventions
See: Table 1
Primary outcome: mean improvement in the maintenance of wakefulness test
This outcome was available in the short‐term for two trials of modafinil accounting for 48 patients (Orlikowski 2009; Talbot 2003). In one cross‐over trial there were 19 participants who had two measurements (Talbot 2003). The mean difference was 2.52 (95% CI: ‐2.32 to +7.37) (random‐effects model) in favor of modafinil, and there was a strong heterogeneity across these studies (I² = 62%) (see Analysis 1.1 and Figure 2).
1.1. Analysis.
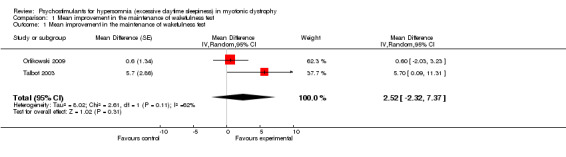
Comparison 1 Mean improvement in the maintenance of wakefulness test, Outcome 1 Mean improvement in the maintenance of wakefulness test.
2.

Forest plot of comparison: 1 Primary outcome: Maintenance of wakefulness test, outcome: 1.1 Mean improvement in the maintenance of wakefulness test.
Neither trial assessed this outcome in the long‐term.
Secondary outcome measures
None of the trials have assessed secondary outcomes in the long‐term. All reported data concerned short‐term treatment effects.
(1) Mean improvement in a sleepiness scale, such as the Epworth Sleepiness Scale
This outcome was available in four trials accounting for 101 patients (MacDonald 2002; Orlikowski 2009; Talbot 2003; Wintzen 2007). In three cross‐over trials, there were respectively, 40 (MacDonald 2002), 20 (Talbot 2003) and 13 (Wintzen 2007) participants who underwent two measurements. The mean difference was ‐2.26 (95% CI ‐3.78 to ‐0.73) (random‐effects model) in favor of modafinil with heterogeneity across the studies (I² = 84%) (Analysis 2.1, and Figure 3 ).
2.1. Analysis.
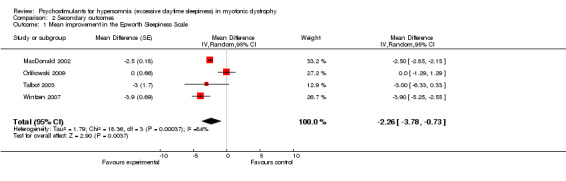
Comparison 2 Secondary outcomes, Outcome 1 Mean improvement in the Epworth Sleepiness Scale.
3.
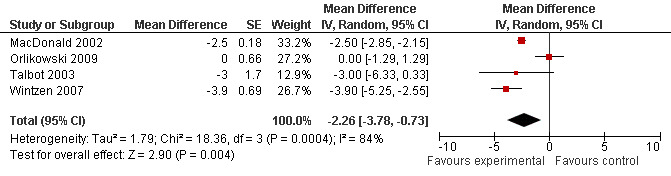
Forest plot of comparison: 2 Secondary outcomes, outcome: 2.1 Mean improvement in the Epworth Sleepiness Scale.
(2) Mean improvement in the multiple sleep latency test
In the randomized cross‐over trial of selegiline (Antonini 1997), there were 11 participants who had two measurements. The multiple sleep latency tests showed a mean difference of ‐3.68 minutes in favor of the control group (95% CI ‐3.99 to ‐3.37) (see Analysis 2.2), which was not significant. This outcome was also available in one trial of modafinil (Orlikowski 2009), the multiple sleep latency tests showed a mean difference of 0.15 (95% CI ‐1.18 to 1.48). The pooled estimate showed a mean difference of ‐1.82 (95% CI ‐5.57 to ‐1.93) (Analysis 2.2 and Figure 4 ).
2.2. Analysis.
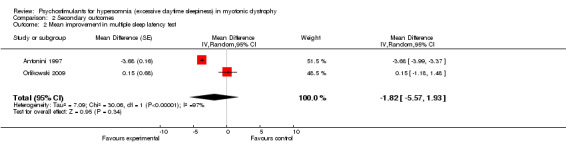
Comparison 2 Secondary outcomes, Outcome 2 Mean improvement in multiple sleep latency test.
4.

Forest plot of comparison: 2 Secondary outcomes, outcome: 2.2 Mean improvement in multiple sleep latency test.
(3) Ambulatory 24 hour sleep‐wake monitoring
We found no randomized trial testing this outcome.
(4) Sleep studies, i.e. apnea‐hypopnea index or mean oxygen saturation or the time spent with an arterial oxygen saturation below 90%
In two randomized trials (Antonini 1997; Talbot 2003), polysomnographic recordings were performed but the results were not available.
(5) Quality of life scale
In one study (MacDonald 2002), the Profile and Mood States scale and the SF 36‐item Health Survey were used. There was no evidence for any substantial benefit from modafinil (Table 2). In two studies (Orlikowski 2009; Talbot 2003), modafinil did not result in any significant changes in the mean improvement in the total score of SF36) (1.27, 95% CI: ‐3.63 to 6.17, random‐effects model) (Analysis 2.3 and Figure 5). In another study, quality of life was assessed using the RAND‐36 scale (Wintzen 2007). There was no evidence for any benefit from modafinil.
1. Quality of life in the MacDonald 2002 study.
| Scales | Mean Delta Placebo | SD Delta Placebo | Mean Delta Modafinil | SD Delta Modafinil | 2 sided P‐values |
| Profile of Mood States (POMS) | |||||
| Anger/Hostility | ‐0.41 | 1.46 | ‐1.25 | 2.98 | 0.15 |
| Confusion/Bewilderment | 0.67 | 1.27 | ‐0.69 | 2.24 | 0.003 |
| Depression/Dejection | ‐0.35 | 2.13 | ‐0.71 | 2.41 | 0.52 |
| Fatigue/Inertia | ‐0.88 | 3.67 | ‐1.85 | 3.77 | 0.29 |
| Tension/Anxiety | ‐0.26 | 1.40 | ‐0.41 | 3.67 | 0.83 |
| Vigour/Activity | 0.62 | 3.67 | ‐0.79 | 3.43 | 0.11 |
| RAND 36‐Item Health Survey Score | |||||
| Physical Functioning | 6.53 | 18.50 | 6.47 | 19.77 | 0.99 |
| Role Limitations Due to Physical Health | 12.18 | 29.70 | 14.62 | 43.79 | 0.78 |
| Role Limitations Due to Emotional Problems | 13.89 | 29.60 | 7.41 | 45.53 | 0.47 |
| Energy/Fatigue | 3.89 | 21.16 | 20.14 | 24.12 | 0.003 |
| Emotional Well Being | 6.78 | 21.43 | 6.42 | 14.98 | 0.94 |
| Social Functioning | 8.03 | 25.41 | 3.48 | 20.49 | 0.45 |
| Pain | 6.67 | 25.14 | 8.89 | 18.66 | 0.68 |
| General Health | 4.19 | 18.36 | 1.81 | 14.35 | 0.55 |
Delta stands for the difference between study day 14 and baseline.
2.3. Analysis.
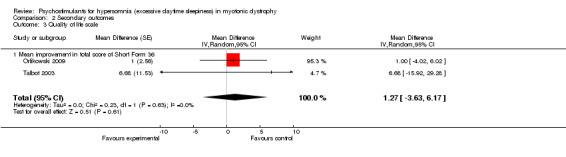
Comparison 2 Secondary outcomes, Outcome 3 Quality of life scale.
5.
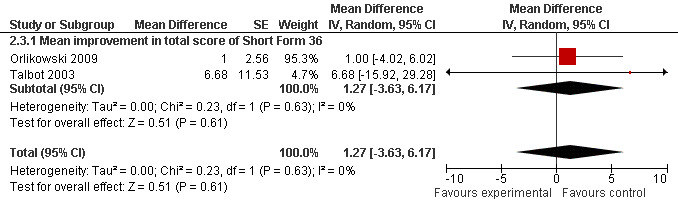
Forest plot of comparison: 2 Secondary outcomes, outcome: 2.3 Quality of life scale.
(6) Adverse events
In one of the randomized trials identified (Antonini 1997), one of the 11 participants dropped out voluntarily because of poor compliance. In the remaining ten, treatment with selegiline was associated with irritability in two participants and slight difficulty in micturition in one male patient. In these participants, side effects never required discontinuation of the study drug or specific treatment.
In the MacDonald 2002 study, 30 participants reported a total of 83 adverse events (65 on modafinil and 18 on placebo), mostly during the first week of treatment. Adverse events that were reported more than once included headache (n = 15), anorexia (n = 6), nausea (n = 6), insomnia (n = 5), anxiety (n = 4), dry mouth (n = 4), dyspepsia (n = 4), dizziness (n = 3), nervousness (n = 3), and tachycardia (n = 2). A total of four randomized participants withdrew from the study, one during the placebo trial in the first week for a reason which was not drug related, and three due to adverse events, one in the placebo group and two in the treated group.
In the third study, no participants withdrew due to adverse events, but one participant withdrew before the final visit for a reason which was not drug related (Talbot 2003).
In the fourth study, the authors did not report any adverse events (Wintzen 2007).
In the fifth study, there were 8 patients (4 in each arm) who experienced at least one adverse event, and no participants withdrew due to adverse events (Orlikowski 2009).
Discussion
Although daytime sleepiness is a common symptom of myotonic dystrophy (Rubinsztein 1998), there have only been five trials that addressed the efficacy of psychostimulants. One failed to demonstrate any beneficial effect of selegiline, a monoamine oxidase B inhibitor. There were several pitfalls in this trial that may not allow one to draw any final conclusion. The trial only included ten evaluable patients. Among these patients presumed to have excessive daytime sleepiness on the basis of a self questionnaire assessment, four patients failed to meet criteria for hypersomnia during the multiple sleep latency tests, i.e. their values for sleep latencies were more than 20 minutes. This highlights the need for a robust diagnostic test of hypersomnia such as the multiple sleep latency test or the maintenance of wakefulness test for both the screening of patients to be enrolled in a trial and the assessment of treatment efficacy. Finally, the dose of selegiline was only 20 mg daily which is less than is commonly used in idiopathic hypersomnia (Hublin 1994).
Four trials have evaluated modafinil (MacDonald 2002; Orlikowski 2009; Talbot 2003; Wintzen 2007). These trials except one (Orlikowski 2009) suggested that modafinil improved patients' sleepiness and was well‐tolerated. Nevertheless, none of these three trials used the conventional maintenance of wakefulness test (Mitler 1982). In one trial the authors used a modified maintenance of wakefulness test in which there were no electroencephalographic recordings and assessment of sleep onset relied on behavioral criteria (Talbot 2003). Patients were asked to tap a proximity detector with a finger repeatedly in response to a dim red light‐emitting diode flashing every three seconds. A failure to respond for 21 seconds was considered as reflecting onset of sleep. This might not be valid in patients with myotonia or cognitive dysfunction. In this study, although post‐treatment absolute values were better in the modafinil group than in the placebo group, the changes from baseline were not significantly different between the two treatment arms for the modified maintenance of wakefulness test, for the Epworth Sleepiness Scale or for the various components of the SF36 scale. The only trial that used conventional maintenance of wakefulness test found no evidence that modafinil significantly improved daytime sleepiness in myotonic dystrophy (Orlikowski 2009).
The meta‐analysis of data from the four trials comparing modafinil to a placebo found no significant benefit from this drug on objective assessment of daytime sleepiness. Nevertheless, there was a significant improvement in patients perception of hypersomnia as indicated by reduction in the score of the Epworth Sleepiness Scale in modafinil‐treated patients (see Table 1). Of note, all these trials were small sized and investigated short‐term effects of treatment on daytime sleepiness.
Authors' conclusions
Implications for practice.
According to low quality evidence, psychostimulants do not improve excessive daytime sleepiness in myotonic dystrophy. Also according to low quality evidence, the only benefit observed with modafinil from multiple measures in adults with myotonic dystrophy, was improved daytime sleepiness as assessed by the Epworth Sleepiness scale. There is no evidence about the efficacy of psychostimulants in children with myotonic dystrophy and excessive somnolence.
Implications for research.
The short‐term and long‐term efficacy and safety of psychostimulants, particularly modafinil, in myotonic dystrophy still remain to be adequately tested in randomized trials.
The primary outcome measure for randomized trials on psychostimulants in myotonic dystrophy should be a reliable index of somnolence, such as the maintenance of wakefulness test.
What's new
| Date | Event | Description |
|---|---|---|
| 24 September 2010 | New search has been performed | We have changed the statistical analysis and used generic inverse variance given that most of trials have a cross‐over design |
| 29 September 2009 | New search has been performed | New searches June 2009. Two new included studies |
History
Protocol first published: Issue 3, 2001 Review first published: Issue 2, 2004
| Date | Event | Description |
|---|---|---|
| 2 July 2008 | Amended | Converted to new review format. |
| 30 April 2006 | New citation required and conclusions have changed | This review was substantively updated in 2006 to incorporate evidence from two new trials. |
Acknowledgements
Our thanks to Philip Barnes, Cardiac/Neuro Care Group, King's College Hospital, London UK, who was co‐author on the protocol, the first published version of this review and the 2006 update.
Appendices
Appendix 1. MEDLINE (OvidSP) Search Strategy
1 randomised controlled trial.pt. 2 controlled clinical trial.pt. 3 randomized.ab. 4 placebo.ab. 5 drug therapy.fs. 6 randomly.ab. 7 trial.ab. 8 groups.ab. 9 or/1‐8 10 exp animals/ not humans.sh. 11 9 not 10 12 (myotoni$ adj5 (dystroph$ or atroph$)).mp. 13 (steinert$ adj5 (disease$ or syndrome$)).mp. 14 Myotonic Dystrophy/ 15 or/12‐14 16 exp Central Nervous System Stimulants/ 17 (central adj5 stimulant$).mp. 18 (psychostimulant$ or caffeine or amphetamine$ or methylphenidate or selegiline or modafinil).mp. 19 or/16‐18 20 exp Disorders of Excessive Somnolence/ 21 (hypersomnia$ or somnolence$ or sleep$ or hypersomn$).mp. 22 exp Sleep Disorders/ 23 or/20‐22 24 11 and 15 and 19 and 23
Appendix 2. EMBASE (OvidSP) Search Strategy
1 crossover‐procedure/ 2 double‐blind procedure/ 3 randomised controlled trial/ 4 single‐blind procedure/ 5 (random$ or factorial$ or crossover$ or cross over$ or cross‐over$ or placebo$ or (doubl$ adj blind$) or (singl$ adj blind$) or assign$ or allocat$ or volunteer$).tw. 6 clinical trial/ 7 or/1‐6 8 animal/ not human/ 9 7 not 8 10 Myotonic Dystrophy/ 11 (myotoni$ adj5 (dystroph$ or atroph$)).mp. 12 (steinert$ adj5 (disease$ or syndrome$)).mp. 13 or/10‐12 14 exp Central Stimulant Agent/ 15 (central adj5 stimulant$).mp. 16 (psychostimulant$ or caffeine or amphetamine$ or methylphenidate or selegiline or modafinil).mp. 17 or/14‐16 18 exp Sleep Disorder/ 19 (hypersomnia$ or somnolence$ or hypersom$ or sleep$).mp. 20 18 or 19 21 9 and 13 and 17 and 20
Appendix 3. Cochrane Central Register of Controlled Trials search strategy
#1myotoni* AND (dystroph* OR atroph*) #2(steinert* AND (disease* or syndrome*)) #3(#1 OR #2) #4MeSH descriptor Central Nervous System Stimulants explode all trees #5CENTRAL NEAR/5 STIMULANT* #6(psychostimulant* OR caffeine OR amphetamine* OR methylphenidate OR selegiline OR modafinil) #7(#4 OR #5 OR #6) #8MeSH descriptor Disorders of Excessive Somnolence explode all trees #9(hypersomnia* OR somnolence* OR sleep* OR hypersomn*) #10MeSH descriptor Sleep Disorders explode all trees #11(#8 OR #9 OR #10) #12(#3 AND #7 AND #11)
Data and analyses
Comparison 1. Mean improvement in the maintenance of wakefulness test.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean improvement in the maintenance of wakefulness test | 2 | Mean Difference (Random, 95% CI) | 2.52 [‐2.32, 7.37] |
Comparison 2. Secondary outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean improvement in the Epworth Sleepiness Scale | 4 | Mean Difference (Random, 95% CI) | ‐2.26 [‐3.78, ‐0.73] | |
| 2 Mean improvement in multiple sleep latency test | 2 | Mean Difference (Random, 95% CI) | ‐1.82 [‐5.57, 1.93] | |
| 3 Quality of life scale | 2 | Mean Difference (Random, 95% CI) | 1.27 [‐3.63, 6.17] | |
| 3.1 Mean improvement in total score of Short Form 36 | 2 | Mean Difference (Random, 95% CI) | 1.27 [‐3.63, 6.17] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Antonini 1997.
| Methods | Single centre, double blind, placebo‐controlled, cross‐over | |
| Participants | n = 11 adults with myotonic dystrophy and excessive daytime sleepiness. One participant dropped out for poor compliance | |
| Interventions | Selegiline 20 mg daily for 30 days, or placebo | |
| Outcomes | Multiple sleep latency test | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | The randomization list was generated by computer |
| Allocation concealment? | Low risk | Coded serial identical containers were used |
| Blinding? All outcomes | Low risk | Identical appearing placebo was used |
| Incomplete outcome data addressed? All outcomes | Low risk | Data are given for all outcomes described in the method section. No loss to follow‐up |
| Free of selective reporting? | Unclear risk | The protocol was not available. Thus it is not clear if reported outcomes were a priori defined and if all a priori defined outcomes were indeed reported |
| Free of other bias? | Low risk | No evidence for protocol violation ‐ the study was not stopped prematurely |
MacDonald 2002.
| Methods | Single centre, double blind, placebo‐controlled, cross‐over | |
| Participants | n = 40 adults with myotonic dystrophy | |
| Interventions | Two periods of two weeks of treatment with modafinil (or a placebo) 100 mg in the morning and 100 mg in the evening for one week, then 200 mg in the morning and 100 mg in the evening for one week | |
| Outcomes | Primary outcome measure: Epworth Sleepiness Scale, Secondary outcome measures: 1) Standford Sleepiness Scale 2) vigour‐activity and fatigue‐inertia factors of the profile of mood states 3) RAND 36‐Item Health Survey 4) voluntary strength 5) adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | The randomization list was generated by computer |
| Allocation concealment? | Low risk | Coded serial identical containers |
| Blinding? All outcomes | Low risk | Indistinguishable placebo was used |
| Incomplete outcome data addressed? All outcomes | Low risk | Data are given for all outcomes described in the method section. No losses to follow‐up |
| Free of selective reporting? | Unclear risk | The protocol was not available. Thus it is not clear if reported outcomes were a priori defined and if all a priori defined outcomes were indeed reported |
| Free of other bias? | Low risk | No evidence for protocol violation ‐ the study was not stopped prematurely |
Orlikowski 2009.
| Methods | Multicentre, randomized placebo‐controlled trial on two parallel groups | |
| Participants | n = 28 adults with myotonic dystrophy with excessive sleepiness assessed by an multiple sleep latency test of less than 8 minutes | |
| Interventions | Modafinil 300 mg daily for one month or its placebo | |
| Outcomes | Primary outcome: Mean improvement in maintenance of wakefulness test Secondary outcomes: (1) Mean latency of multiple sleep latency test (2) Epworth Sleepiness Scale (3) Global patients self assessment and physician assessment of therapeutic effect using a visual analogue scale (VAS) graduated from 0 (no effect) to 100 (full recovery from excessive daytime sleepiness) (4) Disturbances of personality and mood as assessed by Hamilton scale (5) Quality of life as assessed by the SF36 scale |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | The randomization list was generated by computer |
| Allocation concealment? | Low risk | Coded serial identical containers were used |
| Blinding? All outcomes | Low risk | Indistinguishable placebo was used |
| Incomplete outcome data addressed? All outcomes | Low risk | Data are given for all outcomes described in the method section. No losses to follow‐up |
| Free of selective reporting? | Low risk | The protocol was available and all comparisons and outcomes reported in the manuscript matched those stated in the protocol |
| Free of other bias? | High risk | The study was interrupted prematurely for slow recruitment rate and drug expiration dates |
Talbot 2003.
| Methods | Single centre, double blind, placebo‐controlled, cross‐over | |
| Participants | n = 20 adults with myotonic dystrophy and an Epworth Sleepiness Scale score of 10 or more | |
| Interventions | Modafinil, single morning dose of 100 mg on days 1 to 5, then 200 mg on days 6 to 28, or placebo | |
| Outcomes | Primary outcome measure: (1) Epworth Sleepiness Scale, (2) Modified maintenance of wakefulness test * Secondary outcome measures: (1) Driving stimulator test + (2) Activity diary (3) Short Form 36 (4) Adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | The randomization list was generated by computer |
| Allocation concealment? | Low risk | Coded serial identical containers were used |
| Blinding? All outcomes | Unclear risk | Indistinguishable placebo was used |
| Incomplete outcome data addressed? All outcomes | Low risk | Data are given for all outcomes described in the method section. None lost to follow‐up |
| Free of selective reporting? | Unclear risk | The protocol was not available. Thus it is not clear if reported outcomes were a priori defined and if all a priori defined outcomes were indeed reported |
| Free of other bias? | Low risk | No evidence for protocol violation ‐ the study was not stopped prematurely |
Wintzen 2007.
| Methods | Single‐centre, randomized cross‐over, placebo‐controlled trial | |
| Participants | n = 13 adults with myotonic dystrophy | |
| Interventions | Two periods of two weeks of treatment with modafinil (or a placebo) 200 mg per day for one week, then either 200 or 400 mg for one week according to patients perception of efficacy and tolerance | |
| Outcomes | Primary outcome: score based on structured interview (range from 0 to 5) Secondary outcomes: RAND‐36; Epworth Sleepiness Scale |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not given in the manuscript |
| Allocation concealment? | Unclear risk | There was no detail on the method used to conceal treatment allocation |
| Blinding? All outcomes | Low risk | Indistinguishable placebo was used |
| Incomplete outcome data addressed? All outcomes | Low risk | Data are given for all outcomes described in the method section. None lost to follow‐up |
| Free of selective reporting? | Unclear risk | The protocol was not available. Thus it is not clear if reported outcomes were a priori defined and if all a priori defined outcomes were indeed reported. |
| Free of other bias? | Low risk | No evidence for protocol violation ‐ the study was not stopped prematurely |
* Modified maintenance of wakefulness test: sleep onset was not assessed by electroencephalographic (as for the conventional test) but by behavioral criteria. + Driving stimulator test: a test of alertness used in studies of obstructive sleep apnea. It uses a computer to simulate car driving at night.
Differences between protocol and review
In the 2010 update, we updated the risk of bias methodology in accordance with the 2008 edition of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2009) and included a 'Summary of findings' table.
Contributions of authors
Potentially relevant papers were scrutinized by two authors (Djillali Annane and Robert Miller) and the selection of eligible studies was agreed by them. Data were extracted by one author (Djillali Annane) and checked by other authors (Dan Moore, Robert Miller). Dan Moore was responsible for statistical analyses from unpublished data provided by primary authors of papers. All authors agreed the final text.
Sources of support
Internal sources
None, Not specified.
External sources
None, Not specified.
Declarations of interest
One of the authors (DA) was an investigator in one of the included trials (Orlikowski 2009) which was funded by the Association Française de lutte contre les Myopathies and by Lafon pharmaceutical company.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Antonini 1997 {published data only}
- Antonini G, Morino S, Fiorelli M, Fiorini M, Giubilei F. Selegiline in the treatment of hypersomnolence in myotonic dystrophy: a pilot study. Journal of Neuroscience 1997;147(2):167‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
MacDonald 2002 {published and unpublished data}
- MacDonald JR, Hill JD, Tarnopolsky MA. Modafinil reduces excessive somnolence and enhances mood in patients with myotonic dystrophy. Neurology 2002;59(12):1876‐80. [DOI] [PubMed] [Google Scholar]
Orlikowski 2009 {published and unpublished data}
- Orlikowski D, Chevret S, Quera Salva MA, Laforêt P, Lofaso F, Verschueren A, et al. Modafinil for the treatment of excessive daytime sleepiness in myotonic dystrophy: a randomised, double‐blind, placebo‐controlled trial. Clinical Therapeutics 2009;31(8):1765‐73. [DOI] [PubMed] [Google Scholar]
Talbot 2003 {published and unpublished data}
- Talbot K, Stradling J, Crosby J, Hilton‐Jones D. Reduction in excess daytime sleepiness by modafinil in patients with myotonic dystrophy. Neuromuscular Disorders 2003;13(5):357‐64. [DOI] [PubMed] [Google Scholar]
Wintzen 2007 {published data only (unpublished sought but not used)}
- Wintzen AR, Lammers GJ, Dijk JG. Does modafinil enhance activity of patients with myotonic dystrophy?: a double‐blind placebo‐controlled crossover study. Journal of Neurology 2007;254(1):26‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Brook 1992
- Brook JD, Currach ME, Harley HG, Buckler AJ, Church D, Aburatini, et al. Molecular basis of myotonic dystrophy expansion of a trinucleotide (CTG) repeat at the 3' end of a protein kinase family member. Cell 1992;68(4):799‐808. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Buxton 1992
- Buxton J, Shelbourne P, Davies J, Jones L, Tongeren T, Aslanidis C, et al. Detection of an unstable fragment of DNA specific to individuals with myotonic dystrophy. Nature 1992;355(6360):547‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bégin 1997
- Bégin P, Mathieu J, Almirall J, Grassino A. Relationship between chronic hypercapnia and inspiratory muscle weakness in myotonic dystrophy. American Journal of Respiratory and Critical Care Medicine 1997;156(1):133‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Carskadon 1986
- Carskadon MA, Dement WC, Mitler MN, Roth R, Westbrook PR, Keenan S. Guidelines for the multiple sleep latency test (MSLT): a standard measure of sleepiness. Sleep 1986;9(4):519‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Damian 2001
- Damian MS, Gerlach A, Schmidt F, Lehmann E, Reichman H. Modafinil for excessive daytime sleepiness in myotonic dystrophy. Neurology 2001;56(6):794‐6. [DOI] [PubMed] [Google Scholar]
Dunne 1994
- Dunne PW, Walch ET, Epstein HF. Phosphorylation reactions of recombinant human myotonic dystrophy protein kinase and their inhibition. Biochemistry 1994;33(35):10809‐14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fu 1992
- Fu YH, Pizzutti A, Fenwick RG Jr, King JR, Rajnarayan S, Dunne PW, et al. An unstable triplet repeat in a gene related to myotonic dystrophy. Science 1992;255(5049):1256‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gillam 1964
- Gillam PMS, Heaf PJD, Kaufman L, Lucas BGD. Respiration in dystrophia myotonica. Thorax 1964;19:112‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gilmartin 1991
- Gilmartin JJ, Cooper BG, Griffiths CJ, Walls TJ, Veale D, Stone TN, et al. Breathing during sleep in patients with myotonic dystrophy and non‐myotonic respiratory muscle weakness. Quarterly Journal of Medicine 1991;78(285):21‐31. [MEDLINE: ] [PubMed] [Google Scholar]
Griggs 1989
- Griggs RC, Wood DS, Working Group on the Molecular Defect in Myotonic Dystrophy. Criteria for establishing the validity of genetic recombination in myotonic dystrophy. Neurology 1989;39(3):420‐1. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Guilleminault 1978
- Guilleminault C, Cummiskey J, Motta J, Lynne‐Davies P. Respiratory and haemodynamic study during wakefulness and sleep in myotonic dystrophy. Sleep 1978;1(1):19‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Harley 1992
- Harley HG, Brook JO, Rundle SA, Crow S, Readon W, Buckler AJ, et al. Expansion of an unstable DNA region and phenotypic variation in myotonic dystrophy. Nature 1992;355(6360):545‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Harper 1979
- Harper P. Myotonic Dystrophy: Major Problems in Neurology. Vol. 9, London: WB Saunders Co, 1979. [Google Scholar]
Higgins 2009
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]. The Cochrane Collaboration, 2009.. Available from www.cochrane‐handbook.org.
Hublin 1994
- Hulin C, Partinen N, Heinonen EH, Puuka P, Salmi T. Selegiline in the treatment of narcolepsy. Neurology 1994;44(11):2095‐101. [DOI] [PubMed] [Google Scholar]
Johns 1991
- Johns MN. A new method for measuring daytime sleepiness: The Epworth Sleepiness Scale. Sleep 1991;14(6):540‐5. [DOI] [PubMed] [Google Scholar]
Mahadevan 1992
- Mahadevan M, Tsilfidis C, Sabourin L, Shutler G, Amemiya C, Jansen G, et al. Myotonic dystrophy mutation: an unstable CTG repeat in the 3' untranslated region of the gene. Science 1992;255(5049):1253‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Manni 1991
- Manni R, Zucca C, Martinetti M, Ottolini A, Lanzi G, Tartara A. Hypersomnia in dystrophia myotonica: a neurophysiological and immunogenetic study. Acta Neurologica Scandinavica 1991;84(6):498‐502. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mitler 1982
- Mitler MM, Gujavarty KS, Browman CP. Maintenance of wakefulness test: a polysomnographic technique for evaluating treatment in patients with excessive somnolence. Electroencephalography Clinical Neurophysiology 1982;53(6):658‐61. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ono 1998
- Ono S, Takahashi K, Jinnai K, Kanda F, Fukuoka Y, Kurisaki H, et al. Loss of serotonin‐containing neurons in the raphe of patients with myotonic dystrophy: a quantitative immunohistochemical study and relation to hypersomnia. Neurology 1998;50(2):535‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rubinsztein 1998
- Rubinsztein JS, Rubinsztein DC, Goodburn S, Holland AJ. Apathy and hypersomnia are common features of myotonic dystrophy. Journal of Neurology, Neurosurgery and Psychiatry 1998;64(4):510‐5. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van der Meché 1994
- Meché FGA, Bogaard JM, Sluys JCM, Schimsheimer RJ, Ververs CCM, Busch HFM. Daytime sleep in myotonic dystrophy is not caused by sleep apnoea. Journal of Neurology, Neurosurgery and Psychiatry 1994;57(5):626‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]


