Discovery and optimization studiesa,b.
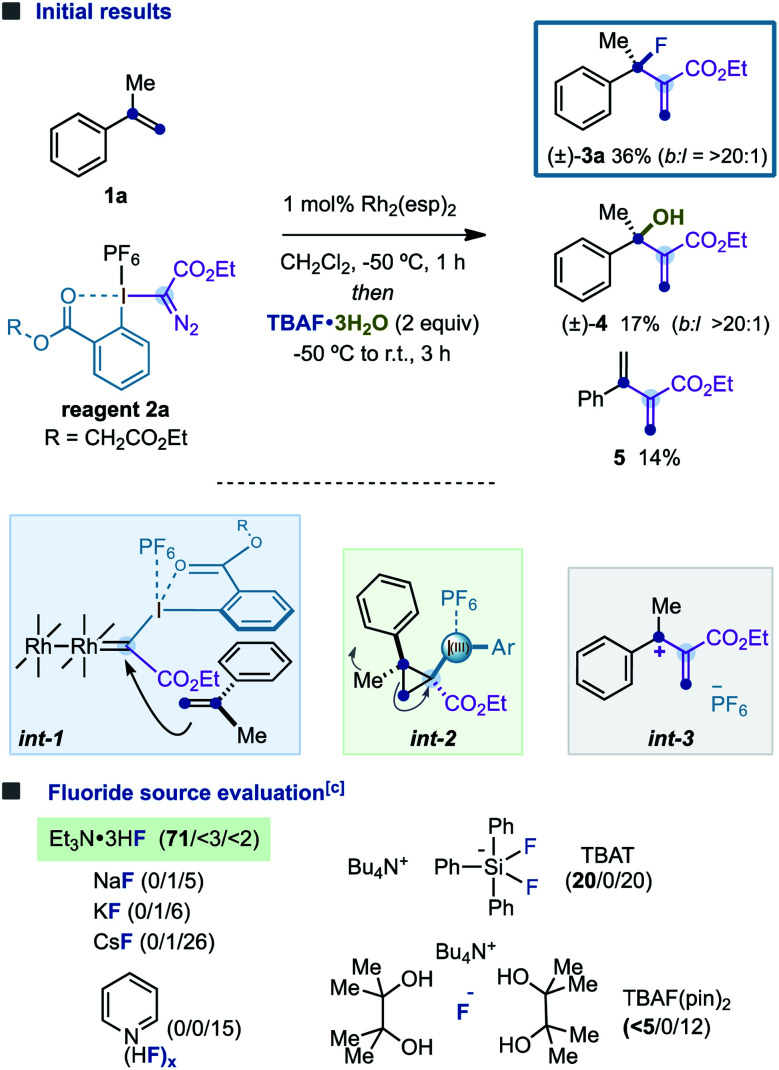
|
Performed with 1a (0.2 mmol, 2 equiv.), 2a (0.1 mmol, 1 equiv.), Rh2(esp)2 (0.002 mmol, 1 mol%), and a fluoride source (3 equiv.) in CH2Cl2 (0.1 M).
Yields are reported on the basis of 1H-NMR analysis using anisole as the internal standard; branched/linear ratio was determined by 19F NMR analysis.
Yields in parentheses are of (±)-3a/(±)-4/5. esp = α,α,α′,α′-tetramethyl-1,3-benzenedipropanoate.
