Synthetic applications.
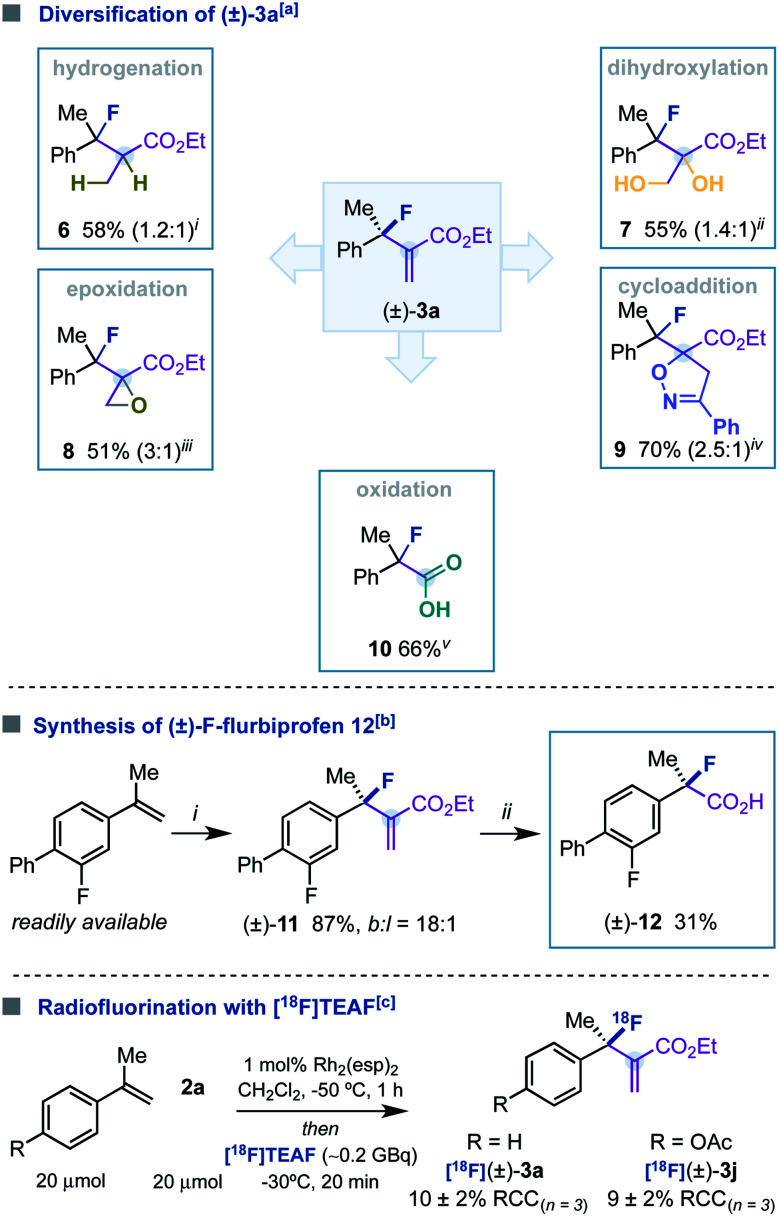
|
Reaction conditions: (i) TsNHNH2, NaOEt, EtOH, 1 hour, and 80 °C; (ii) OsO4 (1 mol%), Oxone, DMF/H2O, 1 hour, and rt; (iii) m-CPBA, CH2Cl2, 14 hours, and reflux; (iv) chlorobenzaldoxime, Et3N, 5 hours, and 0 °C; (v) OsO4 (1 mol%), Oxone, DMF, 14 hours, and rt; then H2O2, 2M NaOH, and THF.
(i) 2-fluoro-4-(prop-1-en-2-yl)-1,1′-biphenyl, Rh2(esp)2 (1 mol%), and Et3N·3HF; (ii) OsO4 (1 mol%), Oxone, DMF, 14 hours, and rt; then H2O2, 2M NaOH, and THF.
1a or 1j (20 μmol), 2a (20 μmol), Rh2(esp)2 (1 mol%), CH2Cl2, 1 hour, and −50 °C; then [18F]TEAF (∼0.2 GBq) in CH2Cl2 (100 μL), 20 min, and −50 °C → −30 °C. RCC was calculated with radio-HPLC with the number of replicates noted.
