Abstract
Background:
Nonalcoholic fatty liver disease (NAFLD) has become one of the leading etiologies of hepatocellular carcinoma (HCC), but risk factors for NAFLD-related HCC occurrence have not been defined. NAFLD is often complicated by metabolic abnormalities, and there is a bidirectional association of metabolic abnormalities with NAFLD progression. This study aimed to systematically evaluate the relationship between metabolic traits and HCC occurrence in patients with NAFLD.
Method:
This study reviewed eight eligible studies that included 297,956 participants, to determine the relationship between metabolic traits and the occurrence of HCC in patients with NAFLD.
Results:
Presence of diabetes mellitus (DM) was associated with increased risk of HCC (HR: 2.65, 95%CI: 2.02 ~ 3.49, Pheterogeneity = 0.589, I2 = 0.0%). Stratified analysis revealed that this risk was higher in NAFLD patients with advanced fibrosis/cirrhosis (HR: 4.55, 95%CI: 2.34 ~ 8.87, Pheterogeneity = 0.870, I2 = 0.0%). Nonetheless even in patients without cirrhosis, DM remained a high risk factor for HCC incidence (HR: 1.80, 95%CI: 1.05 ~ 3.06, Pheterogeneity = 0.291, I2 = 10.4%). Overweight/obesity had a slight correlation with increased risk of HCC occurrence in NAFLD patients (HR: 1.31, 95%CI: 1.00 ~ 1.71, Pheterogeneity = 0.888, I2 = 0.0%), while presence of hypertension and dyslipidemia had no correlation.
Conclusion:
DM and overweight/obesity are high risk factors for NAFLD-related HCC. In particular, DM increases 4-fold the risk of HCC incidence in NAFLD patients with advanced fibrosis/cirrhosis. There is a need to strengthen surveillance for HCC in NAFLD patients with DM, especially in those with advanced fibrosis/cirrhosis.
Keywords: Diabetes mellitus, dyslipidemia, hepatocellular carcinoma, hypertension, nonalcoholic fatty liver disease, obesity
INTRODUCTION
Hepatocellular carcinoma (HCC) is the most common primary liver malignancy, the fifth most common cancer and the second leading cause of cancer-related mortality worldwide.[1,2] Chronic liver diseases such as hepatitis B virus (HBV), hepatitis C virus (HCV), and alcoholic liver disease are the most common causes of HCC. Nonalcoholic fatty liver disease (NAFLD) is one of the most common chronic liver diseases with a spectrum that ranges from simple steatosis to nonalcoholic steatohepatitis, with or without fibrosis.[3] Since viral hepatitis is preventable and treatable, NAFLD has gradually become a leading etiology of HCC.[4]
Clinical guidelines recommend cirrhotic patients be monitored every six months with abdominal ultrasonography, for occurrence of HCC.[5] Nonetheless, abdominal ultrasonography is limited by its low sensitivity and difficult definition of high-risk patients, therefore, most of those with NAFLD-related HCC are diagnosed at an advanced stage of disease (Barcelona Clinic Liver Cancer stage C or D), with a median survival time less than 11 months.[6] It is critical to identify risk factors for HCC progression in NAFLD. NAFLD is often complicated by metabolic abnormalities such as type 2 diabetes mellitus (T2DM) and metabolic syndrome (MetS). Metabolic abnormalities aggravate liver histological lesions in NAFLD, and in turn NAFLD presence increases the incidence of metabolic disorders and is correlated with increased risk of fatal cardiovascular disease (CVD).[7] Experiments have confirmed that NAFLD in the presence of metabolic stress can progress to HCC.[8] Lipotoxicity, chronic inflammation and gut dysbiosis are involved in the process of hepatocarcinogenesis in NAFLD.[9,10] Based on the above, metabolic abnormalities may be high risk factors for the occurrence of NAFLD-related HCC. Specific metabolic traits that are related to the occurrence of NAFLD-related HCC require systematic evaluation.
According to the results of previous clinical studies, the relationship between metabolic abnormalities and the occurrence of NAFLD-HCC remains controversial. Studies by Bertot et al.[11,12,13,14,15] indicated that DM was an independent risk factor for HCC incidence in NAFLD patients, but Grimaudo et al.[16,17,18] reported conflicting results. We conducted this systematic review and meta-analysis to assess the correlation of metabolic traits, including DM, overweight/obesity, hypertension and dyslipidemia, with NAFLD-related HCC incidence.
METHODS
Search strategy
Two authors (Jin Chen and Shu Song) independently searched for published articles in PubMed, Embase, and Cochrane databases up to and including April 2021, with no start date limit, using the following search terms: “diabetes”, “dyslipidemia”, “hyperlipidemia”, “hypertension”, “overweight”, “obesity” and “nonalcoholic fatty liver disease”. Searches were limited to English publications and clinical studies. When population or results were investigated repeatedly in two or more studies, only the most recent and complete study was included. This study was conducted according to the PRISMA guidelines.[19]
Eligibility criteria and quality assessment
Studies were considered eligible for inclusion if they met the following criteria: 1) population-based longitudinal cohort study, 2) subjects were NAFLD patients, 3) exposure factors were defined at baseline: impaired fasting glucose regulation (IFG)/T2DM/DM (unspecified), overweight/obesity, dyslipidemia/hyperlipidemia/hypercholesterolemia/hyperlipoproteinemia, 4) definitive outcome during follow-up i.e., number of HCC cases, and 5) hazard ratios (HRs) calculated by Cox or competing risk regression. Studies were excluded if they were: 1) an animal study, 2) cross-sectional studies, 3) a systematic review or meta-analyses, 4) conference abstract, meeting proceedings, and letters to the editor, 5) had incomplete data, or 6) any research that was not related to the subject of this analysis. The quality of the included studies was assessed by the Newcastle-Ottawa scale[20] with the score ranging from 0 to 9, and comprised of a description of the participants selection (0-4), comparability of the groups (0-2), and ascertainment of outcomes (0-3).
Data extraction
The following data were extracted independently from original studies by two investigators (Jin Chen and Shu Song): the first author's name, publication year, study country, study design, sample size, follow-up, outcome during follow-up, exposure factors, HRs for assessing the relationship between exposure factors and the outcome. In this study, we unified IFG/T2DM, T2DM and DM (unspecified) as DM, and dyslipidemia, hyperlipidemia, hypercholesterolemia, hyperlipoproteinemia as dyslipidemia. If there was disagreement on extracted data, a third investigator was consulted to resolve the inconsistency.
Statistical analysis
This meta-analysis was performed using STATA 14.0 software (College Station, TX, USA). The association between metabolic traits and NAFLD-related HCC incidence was estimated by using hazard ratios (HRs), with 95% confidence interval (95% CI). Pooled HRs were calculated through a random effects model (inverse-variance model), and a P value < 0.05 was considered statistically significant. Heterogeneity was assessed by the Q test and Higgins's inconsistency index (I2), and a P value < 0.05 or an I2 value exceeding 50% indicated significant heterogeneity. Sensitivity analyses were performed by removing studies one by one from the meta-analysis to assess the stability of results. Begg's and Egger's tests were used to evaluate publication bias, and a symmetrical funnel plot and P value > 0.05 indicated that publication bias in this meta-analysis was not significant.
RESULTS
Eight longitudinal cohort studies,[11,12,13,14,15,16,17,18] containing a total of 297,956 NAFLD patients with or without metabolic traits, were included in this meta-analysis [Figure 1]. All investigated the association between DM and HCC incidence, four evaluated the association of overweight/ obesity with HCC incidence, four estimated the association between hypertension and HCC occurrence, and five studied the association between dyslipidemia and HCC incidence in NAFLD patients. The mean/median follow-up ranged from 46 months to 9.3 years, and patient numbers for HCC occurrence during follow-up ranged from 7 to 253. The included studies were all high-quality longitudinal cohort studies and no low-quality studies were identified for inclusion [Tables 1 and 2].
Figure 1.
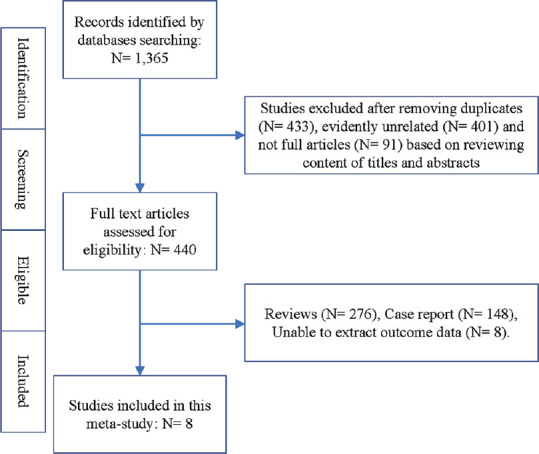
Flow chart of this study design
Table 1.
Characteristics of included studies and quality assessment
| Authors & Years | Country | Study Design | Sample Size (n) | NAFLD definition | Advanced fibrosis/cirrhosis definition | Follow-up | HCC presence (n) | Metabolic traits | Research qualitya |
|---|---|---|---|---|---|---|---|---|---|
| Bertot, 2018 | Australia | Prospective longitudinal cohort study | NAFLD: 284 | Liver biopsy showing >5% steatosis or fatty infiltration confirmed by imaging and excluding other types of liver diseases | Advanced fibrosis: fibrosis stage 3-4 confirmed by liver biopsy; Cirrhosis: fibrosis stage 4 by liver biopsy | 51 months (median) | 28 | T2DM | 8 |
| Kanwal, 2020 | USA | Retrospective longitudinal cohort study | NAFLD: 271,906; NAFLD without cirrhosis: 271,906 | Elevated ALT values appearing more than twice, with more than 6 months apart, and excluding other types of liver diseases | Cirrhosis: ≥2 outpatient or ≥1 inpatient ICD-9 code | 9.3 years (mean) | 253 (NAFLD); 64 (NAFLD without cirrhosis) | T2DM; Obesity (BMI >30 kg/m2); Dyslipidemia; Hypertension | 9 |
| Yang, 2020 | USA | Prospective longitudinal cohort study | NAFLD with cirrhosis: 354 | Clinical, radiologic or histologic evidence of fatty liver disease or cryptogenic liver disease with metabolic syndrome in the absence of other causes | Cirrhosis: liver histology, features of portal hypertension or radiographical evidence (nodular contour of the liver or increased liver stiffness >5 kPa measured by MR elastography) | 46-47 months (median). | 30 | DM (unspecified); Hyperlipidemia; Hypertension | 7 |
| Grimaudo, 2020 | Italy | Prospective longitudinal cohort study | NAFLD: 471 | The presence of ultrasonography-assessed steatosis plus at least one criterion of the metabolic syndrome and excluding other types of liver diseases | Cirrhosis: liver histology or liver stiffness measurement >11.5 KPa for M probe or >11 KPa for XL probe | 64.6 months (median) | 13 | IFG/T2DM; Obesity (BMI ≥30 kg/m2) | 9 |
| Lee, 2017 | China Taiwan | Retrospective longitudinal cohort study | NAFLD without cirrhosis: 18,080 | ICD-9 code: 571.8 and excluding other types of liver diseases based on ICD-9 code | Not included | 6.32 years (median) | 41 | T2DM; Hypercholesterolemia; Hypertension | 8 |
| Ito, 2020 | Japan | Prospective longitudinal cohort study | NAFLD: 179 | Fatty changes in the liver observed by imaging and excluding other types of liver diseases | Cirrhosis: fibrosis stage 4 confirmed by liver biopsy | 7.9 years (median) | 7 | DM (unspecified); Overweight (BMI ≥25 kg/m2); Dyslipidemia; Hypertension | 7 |
| Kawamura, 2012 | Japan | Retrospective longitudinal cohort study | NAFLD: 6,508 | Ultrasonography finding of bright liver with stronger echoes in the hepatic parenchyma than in the renal or spleen parenchyma and excluding other types of liver diseases | Cirrhosis: fibrosis stage 4 confirmed by liver biopsy | 2,051 days (median) | 16 | T2DM; Overweight (BMI ≥25 kg/m2); LDL cholesterol ≥140mg/dl | 8 |
| Vilar-Gomez, 2018 | Europe, Asia, Australia and USA | Prospective longitudinal cohort study | NAFLD with advanced fibrosis (F3/F4): 458 | Liver biopsy showing >5% steatosis and excluding other types of liver diseases | Advanced fibrosis: fibrosis stage 3-4 confirmed by liver biopsy; Cirrhosis: fibrosis stage 4 confirmed by liver biopsy | 5.5 years (mean) | 41 | T2DM | 8 |
Note: a: Research quality was assessed by Newcastle-Ottawa scale and the score ranged from 0 to 9 points. USA: United States of America, NAFLD: Nonalcoholic fatty liver disease, HCC: Hepatocellular carcinoma, T2DM: Type 2 diabetes mellitus, BMI: Body mass index, LDL: Low density lipoprotein
Table 2.
Association between metabolic traits and NAFLD-related HCC occurrence in included studies
| Authors & Years | HRs calculation | Outcome | Exposure factors | Adjusted factors | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| DM | Over weight or obesity | Dyslipidemia | Hypertension | ||||
| Bertot, 2018 | Cox regression | HCC | In all NAFLD patients: T2DM 2.9 95% CI (1.2-7.3) | Not mentioned | Not mentioned | Not mentioned | Not mentioned |
| Kanwal, 2020 | Cox regression | HCC | In all NAFLD patients: T2DM 2.77 95% CI (2.03-3.77); In NAFLD patients without cirrhosis: T2DM 2.15 95% CI (1.20-3.85), | In all NAFLD patients: Obesity 1.31 95% CI (0.98-1.74); In NAFLD patients without cirrhosis: Obesity 1.19 95% CI (0.69-2.07) | In all NAFLD patients: Dyslipidemia 1.31 95% CI (0.84-2.04); In NAFLD patients without cirrhosis: Dyslipidemia 1.73 95% CI (0.72-4.12) | In all NAFLD patients: Hypertension 1.25 95% CI (0.65-2.42); In NAFLD patients without cirrhosis: Hypertension 0.78 95% CI (0.27-2.27) | Age, Gender, Race, T2DM, Obesity (BMI >30 kg/m2), Dyslipidemia, Hypertension |
| Yang, 2020 | Cox regression | HCC | In NAFLD patients cirrhosis: DM 4.18 95% CI (1.23-14.2) | Not mentioned | In NAFLD patients cirrhosis: Hyperlipidemia 0.98 95% CI (0.43-2.20) | In NAFLD patients cirrhosis: Hypertension 0.67 95% CI (0.30-1.46) | Only the association between DM and HCC was adjusted by age and albumin. |
| Grimaudo, 2020 | Cox regression | HCC | In all NAFLD patients: IFG/T2DM 2.9 95% CI (1.2-7.3) | In all NAFLD patients: Obesity 0.87 95% CI (0.21-3.56) | Not mentioned | Not mentioned | Age, Obesity (BMI ≥30 kg/m2), PLT, Albumin, IFG/T2DM, PNPLA3 polymorphism, Advanced Fibrosis/Cirrhosis |
| Lee, 2017 | Cox regression | HCC | In NAFLD patients without cirrhosis: T2DM 1.19 95% CI (0.47-3.02) | Not mentioned | In NAFLD patients without cirrhosis: Hypercholesterolemia 0.41 95% CI (0.15-1.11) | In NAFLD patients without cirrhosis: Hypertension 1.14 95% CI (0.46-2.80) | Age, Gender, ALT, Hypertension, Hypercholesterolemia, T2DM, Gout, Statin use, Metformin use, Aspirin use |
| Ito, 2020 | Cox regression | HCC | In all NAFLD patients: DM 1.832 95% CI (0.354-9.469) | In all NAFLD patients: Overweight 1.068 95% CI (0.207-5.508), | In all NAFLD patients: Dyslipidemia 0.213 95% CI (0.048-0.953), | In all NAFLD patients: Hypertension 2.810 95% CI (0.545-14.490) | Unadjusted |
| Kawamura, 2012 | Cox regression | HCC | In all NAFLD patients: T2DM 3.21 95% CI (1.09-9.50) | In all NAFLD patients: Overweight 1.69 95% CI (0.63-4.55) | In all NAFLD patients: LDL cholesterol≥140mg/dl 1.07 CI (0.40-2.89) | Not mentioned | Only the association between T2DM and HCC was adjusted by Age, ALT and PLT |
| Vilar-Gomez, 2018 | Competing risk regression | HCC | NAFLD patients with advanced fibrosis: T2DM 4.72 95% CI (2.13-10.45) | Not mentioned | Not mentioned | Not mentioned | Cirrhosis, Gender, Race, Age, Smoking, BMI, Hypertension, History of vascular events or malignant neoplasm, Statin therapy, Glucose-lowering medications, Anti-hypertensive medications, Aspirin, INR, Albumin, Total bilirubin, AST/ALT, PLT, MELD score |
HCC: Hepatocellular carcinoma, NAFLD: Nonalcoholic fatty liver disease, T2DM: Type 2 diabetes mellitus, IFG: Impaired fasting glucose regulation, BMI: Body mass index, LDL: Low density lipoprotein, PLT: Platelet, ALT: Alamine aminotransferase, AST: Aspartate transaminase
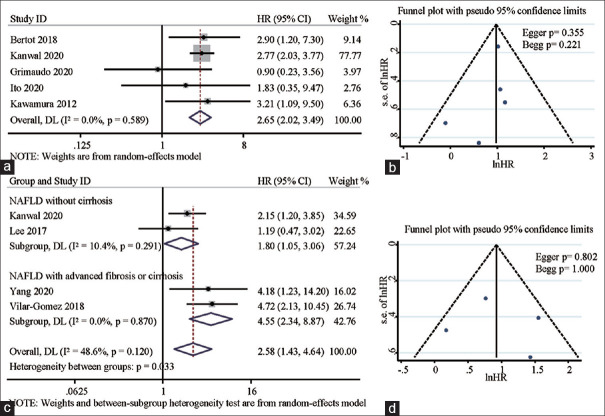
The likelihood was that HCC incidence in NAFLD patients with DM was significantly higher than that in patients without DM, with a pooled HR of 2.65 (95%CI: 2.02 ~ 3.49). The statistical heterogeneity was insignificant (Pheterogeneity = 0.589), with an I2 of 0.0% [Figure 2a]. Moreover, stratified analysis based on NAFLD severity revealed that DM presence was associated with high incidence of HCC, regardless of the presence of cirrhosis: pooled HR in patients without cirrhosis was 1.80 (95%CI: 1.05 ~ 3.06, Pheterogeneity = 0.291, I2 = 10.4%) and pooled HR in patients with advanced fibrosis/ cirrhosis was 4.55 (95%CI: 2.34, 8.87, Pheterogeneity = 0.870, I2 = 0.0%) [Figure 2c].
Figure 2.
Forest plots depicting the correlation between DM and NAFLD-related HCC incidence (a), and correlation of DM with HCC incidence in noncirrhotic and cirrhotic NAFLD (b). Funnel plots depicting potential publication bias in pooled HRs of the association between DM and NAFLD-related HCC incidence (c), and pooled HRs of the association between DM and HCC incidence in noncirrhotic and cirrhotic NAFLD (d)
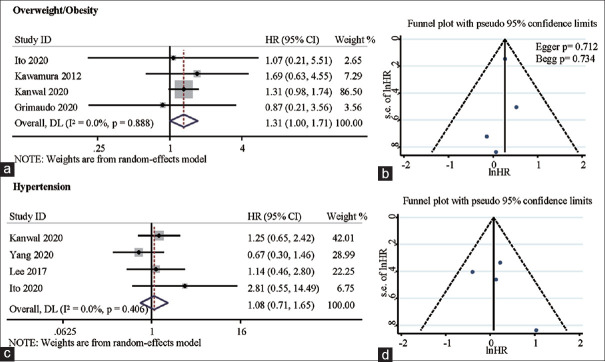
The relationship between presence of overweight/obesity and hypertension, and NAFLD-related HCC incidence is shown in Figure 3. Overweight/obesity NAFLD patients were at higher risk of developing HCC than those without overweight/obesity, with a pooled HR of 1.31 (95%CI: 1.00 ~ 1.71, Pheterogeneity = 0.888, I2 = 0.0%) [Figure 3a]. Nonetheless our results showed that hypertension presence was not associated with the risk of NAFLD-related HCC incidence, with a pooled HR of 1.08 (95%CI: 0.71 ~ 1.65, Pheterogeneity = 0.406, I2 = 0.0%) [Figure 3c].
Figure 3.
Forest plots depicting the correlation between overweight/obesity and NAFLD-related HCC incidence (a), and correlation between hypertension and NAFLD-related HCC incidence (b). Funnel plots depicting potential publication bias in pooled HRs of the association between overweight/obesity and NAFLD-related HCC incidence (c), and pooled HRs of the association between hypertension and NAFLD-related HCC incidence (d)
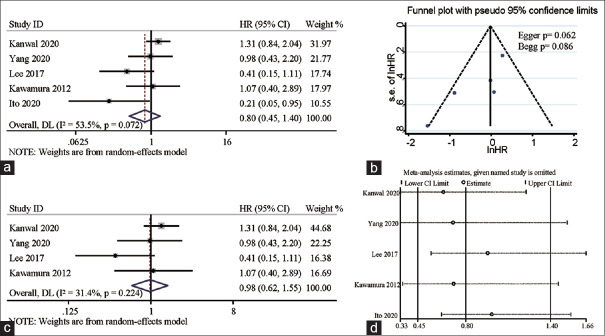
We also evaluated the relationship between presence of dyslipidemia and NAFLD-related HCC. As shown in Figure 4a, dyslipidemia in NAFLD patients had no correlation with HCC development: pooled HR was 0.80 (95%CI: 0.45 ~ 1.40, Pheterogeneity = 0.072, I2 = 53.5%). Since there was a high heterogeneity in the results, we deleted the study with the largest heterogeneity but the result (HR: 0.98, 95%CI 0.62-1.55, Pheterogeneity = 0.224, I2 = 31.4%) was consistent with the previous one [Figure 4c]. Furthermore, we conducted sensitivity analysis by eliminating included studies one by one, and the result was again consistent with the previous result [Figure 4d].
Figure 4.
Forest plots depicting the correlation between dyslipidemia and NAFLD-related HCC incidence (a). Funnel plots depicting potential publication bias in pooled HRs of the association between dyslipidemia and NAFLD-related HCC incidence (b), and results from the sensitivity analysis of pooled HRs of the association between dyslipidemia and NAFLD-related HCC incidence (c and d)
Finally, we investigated publication bias in these results. As shown in Figures 2b, 2d, 3b, 3d and 4b, funnel plots of all pooled HRs were morphologically symmetrical and there was no significant difference in any results on Begg and Egger tests.
DISCUSSION
In recent years, the increasing incidence of NAFLD and absence of treatment approaches have led to a gradual increase in patients with NAFLD-related HCC.[21] Identification of the population of patients with NAFLD at high risk of developing HCC is vital. Nonetheless known risk factors for HCC are not completely applicable in such patients.[6] Metabolic abnormalities are not only common complications of NAFLD, but also positively correlated with the severity of histological injury.[22] In this systematic review and meta-analysis, DM and overweight/obesity were associated with an increased risk of HCC incidence in NAFLD patients, especially DM, when the risk increased 4-fold if advanced fibrosis/cirrhosis was also present.
The first important finding was that DM and overweight/obesity were both risk factors for HCC incidence in NAFLD. In noncirrhotic and cirrhotic NAFLD patients, DM remained a high risk factor. Streptozotocin (STZ), a chemical agent that destroys pancreatic β cells and causes increased blood glucose, has been proven to induce NAFLD-related HCC in rodents fed a high fat diet (HFD).[8] This indirectly suggests that elevated blood glucose might be a key factor in the development of NAFLD-related HCC. An increasing number of studies has shown that reprogramming of glucose metabolism is crucial for tumor formation and development, including HCC.[23,24] Glucose is the most important nutrient in vivo, and in HCC development, insufficient glucose supply can induce hypoxia and up-regulation of hypoxia inducible factor (HIF) signal, further activating oncogenes and deactivating tumor suppressor genes.[25] This hypoxia signal is also involved in tumor epithelial mesenchymal transition (EMT), metastasis and other biological behaviors. Glucose uptake and utilization in the peripheral tissues of patients with DM has been shown to be impaired and may be an important reason for the hypoxic environment and NAFLD-related HCC development.
The relationship between obesity and tumorigenesis has been reported by many studies.[26] In NAFLD patients, obesity is also associated with more severe histological injury.[22] Obesity is characterized by the expansion of adipose tissue, and often accompanied by inflammation of adipose tissue. Studies have shown that peritumoral adipocytes, also known as cancer-associated adipocytes (CAAs), interact directly with cancer cells to promote tumor growth. CAAs have been shown to secrete numerous proteases and cytokines, including IL-6 and IL-8, to promote tumor growth, invasion and migration.[27] This suggests that obesity might participate in the development of NAFLD-related HCC by inducing adipose tissue inflammation and CAA formation. Our findings showed that obesity was a risk factor for NAFLD-related HCC, but the impact of overweight/obesity on NAFLD-related HCC occurrence was mild compared with that of DM. In this study, overweight/obesity was measured by BMI. A previous large-scale clinical study revealed that BMI was associated with HCC incidence, with a modest HR of 1.19.[28] This modest risk was likely due to the limitations of BMI in assessment of overweight/obesity. Although BMI is the easiest and most available clinical measure for body/obesity types, it is not able to differentiate fat and skeletal muscle mass.[29] Unlike fat, skeletal muscle plays a protective role in cancer progression. Previous studies showed that skeletal muscle loss was not only associated with increased incidence of HCC, but also served as a poor prognostic marker of HCC.[30] Skeletal muscle, as an endocrine organ, can secrete a series of myokines such as irisin and IL-15 to inhibit cancer development.[31] Therefore, a more comprehensive assessment of body/obesity types is needed to clarify the relationship between obesity and NAFLD-related HCC occurrence.
Another interesting finding of this study was that hypertension and dyslipidemia have no significant correlation with the occurrence of HCC in NAFLD patients. Hypertension and dyslipidemia were recognized as high risk factors for cardiovascular events, but their correlation with tumorigenesis remains controversial. In kidney cancer, Flaherty et al.[32] reported that hypertension was not associated with cancer incidence, while Chow et al.[33] suggested that elevated blood pressure was associated with an increased risk of cancer. The same contradictory results were evident when assessing the relationship between dyslipidemia and tumorigenesis.[34,35] Our results indicated that these two factors were not related to NAFLD-related HCC incidence.
Compared with other types of liver disease, surveillance for HCC in NAFLD patients is difficult. A large UK cohort study showed that 56.7% of patients with NAFLD-related HCC had not been monitored prior to diagnosis, compared with only 13.3% of those with HCV-related HCC.[36] Another multicenter study indicated that the detection rate of HCC by ultrasonic surveillance in NAFLD was much lower than that in HCV.[37] This is probably due to the large population base of patients with NAFLD and the lack of reliable methods to stratify their risk of HCC. AASLD guidelines categorised HCC surveillance for NAFLD-related cirrhosis under ‘’other conditions”, with no specific recommendations.[38] EASL-EASD-EASO clinical practice guidelines concluded that surveillance for HCC in NAFLD was difficult to implement since high risk patients were not clearly identified.[39] Obesity, DM, hypertension and hyperlipidemia are common comorbidities in NAFLD and might have a causal relationship.[7] Whether these comorbidities can increase HCC risk is nonetheless unclear. Our findings emphasized the contribution of DM to NAFLD-related HCC and suggest that diabetic NAFLD patients constitute a high risk population for NAFLD-related HCC, especially in the presence of advanced fibrosis/cirrhosis. This provides supplementary knowledge for risk stratification and monitoring of NAFLD-related HCC.
The assessment of heterogeneity was an important part of the meta-analysis. Significant heterogeneity existed when assessing the relationship between dyslipidemia and NAFLD-related HCC incidence. This heterogeneity was mainly caused by the inclusion of Ito's study although removal of this study and performing sensitivity analysis did not alter our conclusion.
This study also had some limitations: metabolic traits often coexisted, but due to the lack of sufficient inclusion studies, we could not assess the additive risk of HCC when NAFLD patients simultaneously suffered from one or more traits. In addition, NAFLD severity was a key factor affecting the occurrence of HCC. In this study, there were insufficient included studies to perform stratified analysis of the relationship between metabolic traits and HCC occurrence according to the severity of NAFLD. Finally, metabolic comorbidities may have been subject to intervention. The lack of certainty about whether metabolic comorbidities were well controlled at baseline may have been an important confounding factor affecting the results. Future research needs to clarify the relationship between the intervention status of metabolic comorbidities and HCC occurrence in patients with NAFLD.
In conclusion, this systematic review and meta-analysis provides evidence that DM and overweight/obesity are risk factors for NAFLD-related HCC incidence. More importantly, DM increased the risk of HCC occurrence 4-fold in cirrhotic NAFLD patients. Since cirrhosis is a risk factor for HCC incidence, diabetic patients with NAFLD and advanced fibrosis/cirrhosis should be monitored closely for development of HCC.
Authors’ contributions
Jin Chen and Shu Song equally contributed to this work. Jin Chen and Shu Song designed the study, analyzed the data and wrote the manuscript. Xiangsu Li and Dongxue Bian designed the search strategy and were involved in data analysis. Xudong Wu was responsible for the research design, data analysis, and manuscript revision. All authors gave their approval for submission of the final manuscript.
Financial support and sponsorship
This study was supported by the Natural Science Foundation of Jiangsu Province (BK20200265) and Medical Research Project of Jiangsu Health Commission (ZDB2020033).
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Lafaro KJ, Demirjian AN, Pawlik TM. Epidemiology of hepatocellular carcinoma. Surg Oncol Clin N Am. 2015;24:1–17. doi: 10.1016/j.soc.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Massarweh NN, El-Serag HB. Epidemiology of hepatocellular carcinoma and intrahepatic cholangiocarcinoma? Cancer Control. 2017;24:1073274817729245. doi: 10.1177/1073274817729245. doi: 10.1177/1073274817729245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diehl AM, Day C. Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. N Engl J Med. 2017;377:2063–72. doi: 10.1056/NEJMra1503519. [DOI] [PubMed] [Google Scholar]
- 4.Baffy G, Brunt EM, Caldwell SH. Hepatocellular carcinoma in non-alcoholic fatty liver disease: An emerging menace. J Hepatol. 2012;56:1384–91. doi: 10.1016/j.jhep.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 5.European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: Management of hepatocellular carcinoma. J Hepatol. 2012;56:908–43. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Anstee QM, Reeves HL, Kotsiliti E, Govaere O, Heikenwalder M. From NASH to HCC: Current concepts and future challenges. Nat Rev Gastroenterol Hepatol. 2019;16:411–28. doi: 10.1038/s41575-019-0145-7. [DOI] [PubMed] [Google Scholar]
- 7.Lonardo A, Nascimbeni F, Mantovani A, Targher G. Hypertension, diabetes, atherosclerosis and NASH: Cause or consequence? J Hepatol. 2018;68:335–52. doi: 10.1016/j.jhep.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 8.Fujii M, Shibazaki Y, Wakamatsu K, Honda Y, Kawauchi Y, Suzuki K, et al. A murine model for non-alcoholic steatohepatitis showing evidence of association between diabetes and hepatocellular carcinoma. Med Mol Morphol. 2013;46:141–52. doi: 10.1007/s00795-013-0016-1. [DOI] [PubMed] [Google Scholar]
- 9.Marra F, Svegliati-Baroni G. Lipotoxicity and the gut-liver axis in NASH pathogenesis. J Hepatol. 2018;68:280–95. doi: 10.1016/j.jhep.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Kanda T, Goto T, Hirotsu Y, Masuzaki R, Moriyama M, Omata M. Molecular Mechanisms: Connections between Nonalcoholic Fatty Liver Disease, Steatohepatitis and Hepatocellular Carcinoma. Int J Mol Sci. 2020;21:1525. doi: 10.3390/ijms21041525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bertot LC, Jeffrey GP, de Boer B, MacQuillan G, Garas G, Chin J, et al. Diabetes impacts prediction of cirrhosis and prognosis by non-invasive fibrosis models in non-alcoholic fatty liver disease. Liver Int. 2018;38:1793–802. doi: 10.1111/liv.13739. [DOI] [PubMed] [Google Scholar]
- 12.Kanwal F, Kramer JR, Li L, Dai J, Natarajan Y, Yu X, et al. Effect of metabolic traits on the risk of cirrhosis and hepatocellular cancer in nonalcoholic fatty liver disease. Hepatology. 2020;71:808–19. doi: 10.1002/hep.31014. [DOI] [PubMed] [Google Scholar]
- 13.Kawamura Y, Arase Y, Ikeda K, Seko Y, Imai N, Hosaka T, et al. Large-scale long-term follow-up study of Japanese patients with non-alcoholic Fatty liver disease for the onset of hepatocellular carcinoma. Am J Gastroenterol. 2012;107:253–61. doi: 10.1038/ajg.2011.327. [DOI] [PubMed] [Google Scholar]
- 14.Yang JD, Ahmed F, Mara KC, Addissie BD, Allen AM, Gores GJ, et al. Diabetes is associated with increased risk of hepatocellular carcinoma in patients with cirrhosis from nonalcoholic fatty liver disease. Hepatology. 2020;71:907–16. doi: 10.1002/hep.30858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vilar-Gomez E, Calzadilla-Bertot L, Wai-Sun Wong V, Castellanos M, Aller-de la Fuente R, Metwally M, et al. Fibrosis severity as a determinant of cause-specific mortality in patients with advanced nonalcoholic fatty liver disease: A multi-national cohort study. Gastroenterology. 2018;155:443–57. doi: 10.1053/j.gastro.2018.04.034. [DOI] [PubMed] [Google Scholar]
- 16.Grimaudo S, Pipitone RM, Pennisi G, Celsa C, Cammà C, Di Marco V, et al. Association between PNPLA3 rs738409 C>G variant and liver-related outcomes in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2020;18:935–44. doi: 10.1016/j.cgh.2019.08.011. [DOI] [PubMed] [Google Scholar]
- 17.Ito T, Ishigami M, Ishizu Y, Kuzuya T, Honda T, Ishikawa T, et al. Serum nutritional markers as prognostic factors for hepatic and extrahepatic carcinogenesis in Japanese patients with nonalcoholic fatty liver disease. Nutr Cancer. 2020;72:884–91. doi: 10.1080/01635581.2019.1653474. [DOI] [PubMed] [Google Scholar]
- 18.Lee TY, Wu JC, Yu SH, Lin JT, Wu MS, Wu CY. The occurrence of hepatocellular carcinoma in different risk stratifications of clinically noncirrhotic nonalcoholic fatty liver disease. Int J Cancer. 2017;141:1307–14. doi: 10.1002/ijc.30784. [DOI] [PubMed] [Google Scholar]
- 19.Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ. 2015;350:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 20.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 21.Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67:123–33. doi: 10.1002/hep.29466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sookoian S, Pirola CJ. Systematic review with meta-analysis: The significance of histological disease severity in lean patients with nonalcoholic fatty liver disease. Aliment Pharmacol Ther. 2018;47:16–25. doi: 10.1111/apt.14401. [DOI] [PubMed] [Google Scholar]
- 23.Hay N. Reprogramming glucose metabolism in cancer: Can it be exploited for cancer therapy? Nat Rev Cancer. 2016;16:635–49. doi: 10.1038/nrc.2016.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Z, Zhang H. Reprogramming of glucose, fatty acid and amino acid metabolism for cancer progression. Cell Mol Life Sci. 2016;73:377–92. doi: 10.1007/s00018-015-2070-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagao A, Kobayashi M, Koyasu S, Chow CC, Harada H. HIF-1-dependent reprogramming of glucose metabolic pathway of cancer cells and its therapeutic significance. Int J Mol Sci. 2019;20:238. doi: 10.3390/ijms20020238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Avgerinos KI, Spyrou N, Mantzoros CS, Dalamaga M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism. 2019;92:121–35. doi: 10.1016/j.metabol.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Iyengar NM, Gucalp A, Dannenberg AJ, Hudis CA. Obesity and cancer mechanisms: Tumor microenvironment and inflammation. J Clin Oncol. 2016;34:4270–6. doi: 10.1200/JCO.2016.67.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhaskaran K, Douglas I, Forbes H, dos-Santos-Silva I, Leon DA, Smeeth L. Body-mass index and risk of 22 specific cancers: A population-based cohort study of 5·24 million UK adults. Lancet. 2014;384:755–65. doi: 10.1016/S0140-6736(14)60892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park J, Ahmadi SF, Streja E, Molnar MZ, Flegal KM, Gillen D, et al. Obesity paradox in end-stage kidney disease patients. Prog Cardiovasc Dis. 2014;56:415–25. doi: 10.1016/j.pcad.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang KV, Chen JD, Wu WT, Huang KC, Hsu CT, Han DS. Association between loss of skeletal muscle mass and mortality and tumor recurrence in hepatocellular carcinoma: A systematic review and meta-analysis. Liver Cancer. 2018;7:90–103. doi: 10.1159/000484950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karstoft K, Pedersen BK. Skeletal muscle as a gene regulatory endocrine organ. Curr Opin Clin Nutr Metab Care. 2016;19:270–5. doi: 10.1097/MCO.0000000000000283. [DOI] [PubMed] [Google Scholar]
- 32.Flaherty KT, Fuchs CS, Colditz GA, Stampfer MJ, Speizer FE, Willett WC, et al. A prospective study of body mass index, hypertension, and smoking and the risk of renal cell carcinoma (United States) Cancer Causes Control. 2005;16:1099–106. doi: 10.1007/s10552-005-0349-8. [DOI] [PubMed] [Google Scholar]
- 33.Chow WH, Gridley G, Fraumeni JF, Jr, Järvholm B. Obesity, hypertension, and the risk of kidney cancer in men. N Engl J Med. 2000;343:1305–11. doi: 10.1056/NEJM200011023431804. [DOI] [PubMed] [Google Scholar]
- 34.Strohmaier S, Edlinger M, Manjer J, Stocks T, Bjørge T, Borena W, et al. Total serum cholesterol and cancer incidence in the Metabolic syndrome and Cancer Project (Me-Can) PLoS One. 2013;8:e54242. doi: 10.1371/journal.pone.0054242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kitahara CM, Berrington de González A, Freedman ND, Huxley R, Mok Y, Jee SH, et al. Total cholesterol and cancer risk in a large prospective study in Korea. J Clin Oncol. 2011;29:1592–8. doi: 10.1200/JCO.2010.31.5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mittal S, Sada YH, El-Serag HB, Kanwal F, Duan Z, Temple S, et al. Temporal trends of nonalcoholic fatty liver disease-related hepatocellular carcinoma in the veteran affairs population. Clin Gastroenterol Hepatol. 2015;13:594–601.e1. doi: 10.1016/j.cgh.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Piscaglia F, Svegliati-Baroni G, Barchetti A, Pecorelli A, Marinelli S, Tiribelli C, et al. Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: A multicenter prospective study. Hepatology. 2016;63:827–38. doi: 10.1002/hep.28368. [DOI] [PubMed] [Google Scholar]
- 38.Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358–80. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 39.European Association for the Study of the Liver (EASL) European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO), EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]