Figure 2.
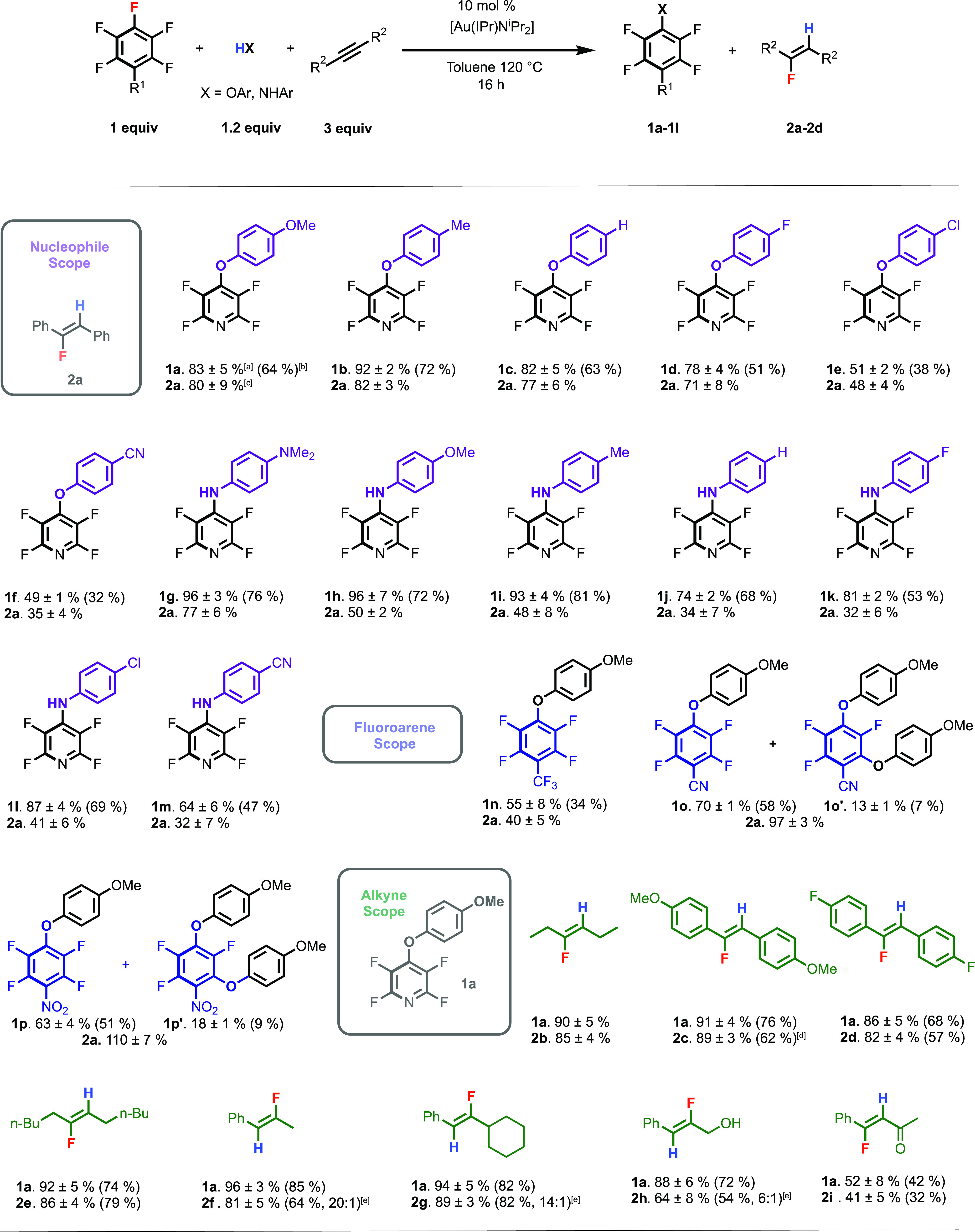
HF transfer reaction scope catalyzed by [Au(IPr)NiPr2]. [a] Reactions were performed with 0.1:1:1.2:3 equivalents of catalyst: fluoroarene (0.04 M): nucleophile: alkyne. Yields of fluoroarene (1a-1p) and fluoroalkene (2a-2d) were calculated from 19F NMR spectroscopy using a fluorobenzene internal standard. Reactions were performed in triplicate, and standard deviations are reported with a 99% confidence level. [b] Isolated yields were obtained from scale-up reactions and are shown in parenthesis. [c] Isolated yields of 2a are not reported due to this compound co-eluting with diphenylacetylene. [d] Due to the challenging isolation, this product was contaminated with ∼20% of unreacted alkyne. [e] Ratio of regioisomers β:α functionalization. Major isomer is shown.
