Scheme 1. Annulation of Oxetanols and 1,2-Diols for the One-Pot Formation of 1,4-Dioxanes.
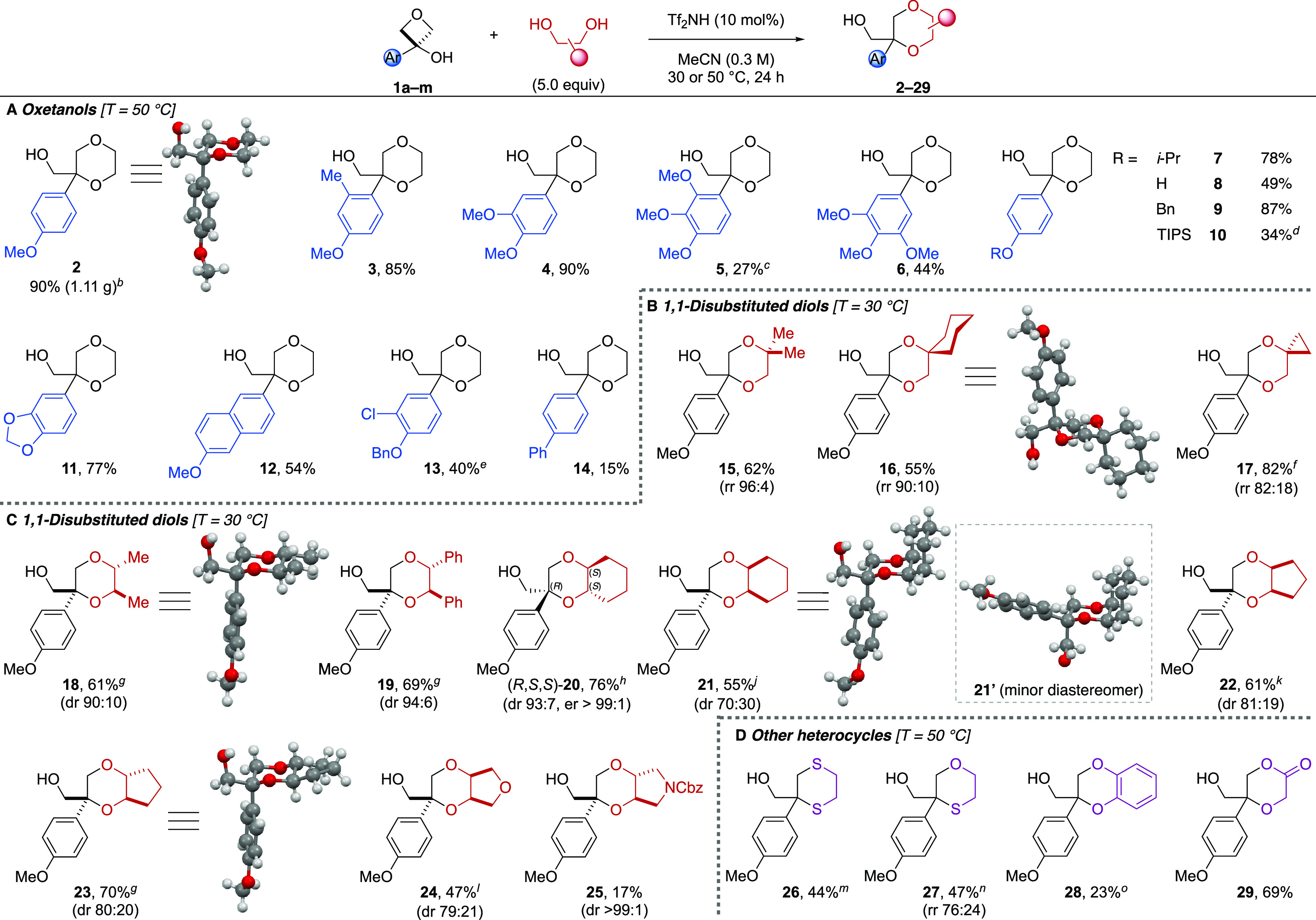
Reactions on a 0.25 mmol scale unless otherwise stated. Isolated yields are reported. Diastereomeric (dr) and regioisomeric (rr) ratios determined from the 1H NMR spectrum of the crude reaction mixture.
Reaction run on a 5.5 mmol scale.
Reaction run on a 0.22 mmol scale.
18% of phenol 8 was also isolated.
Reaction run for 32 h.
Reaction run on a 0.136 mmol scale and the product isolated as a mixture of regioisomers with the indicated rr.
Product isolated as a mixture of diastereomers with the indicated dr.
An additional 11% of a diastereomeric mixture was isolated (dr 67:33).
Reaction run at 50 °C.
Additional 10% of a diastereomeric mixture was isolated (dr 26:74).
Additional 20% of a diastereomeric mixture was isolated (dr 39:61).
Reaction run at 0–30 °C and using 1.2 equiv of bis-nucleophile (see Supporting Information Table S3).
Reaction run on a 0.38 mmol scale (oxetanol) at 0–23 °C and using 0.75 equiv of bis-nucleophile (see Supporting Information Table S4). Yield based on bis-nucleophile.
Using TfOH (5 mol %) in CHCl3 (0.5 M) at 25 °C (see Supporting Information Table S5).
