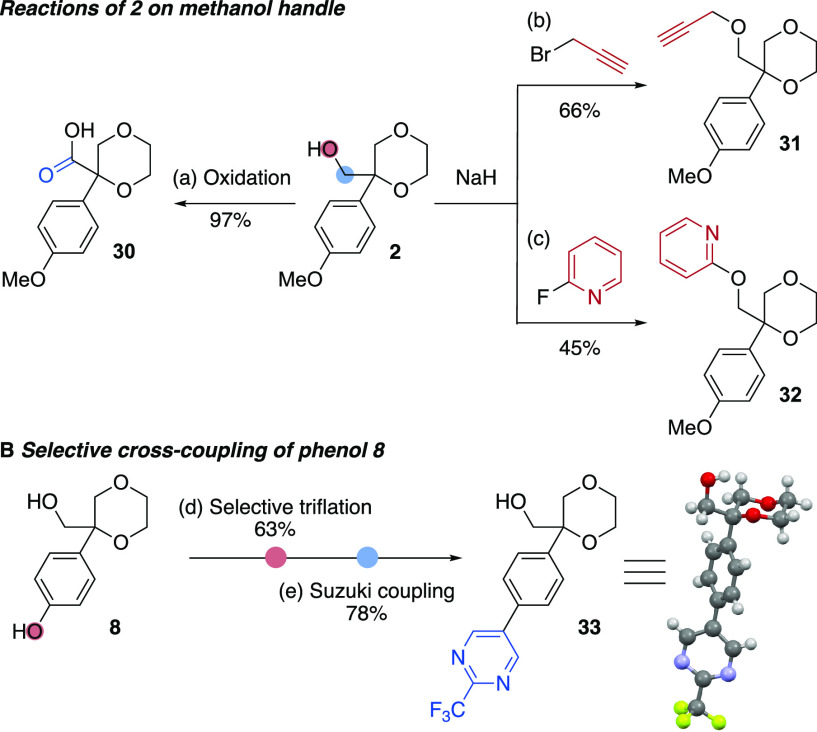
Scheme 2. Derivatizations of 1,4-Dioxane Products.
Reactions on a 0.2 mmol scale. Isolated yields are reported. Conditions: (a) aq. KMnO4, 1 M aq. NaOH, 0–25 °C, 4 days. (b) Propargyl bromide (2.0 equiv), NaH (5.0 equiv), DMF, 0–25 °C, 19 h. (c) 2-Fluoropyridine (1.33 equiv), NaH (1.2 equiv), DMF, 0–90 °C, 24 h. (d) NTf2Ph (1.5 equiv), NEt3 (3.0 equiv), DMAP (10 mol %), CH2Cl2, 0–25 °C, 4 h. (e) Ar–Bpin (1.5 equiv), Pd(OAc)2 (5 mol %), SPhos (10 mol %), K3PO4 (2.0 equiv), dioxane/H2O (4:1), 65 °C, 44 h.