FIGURE 1.
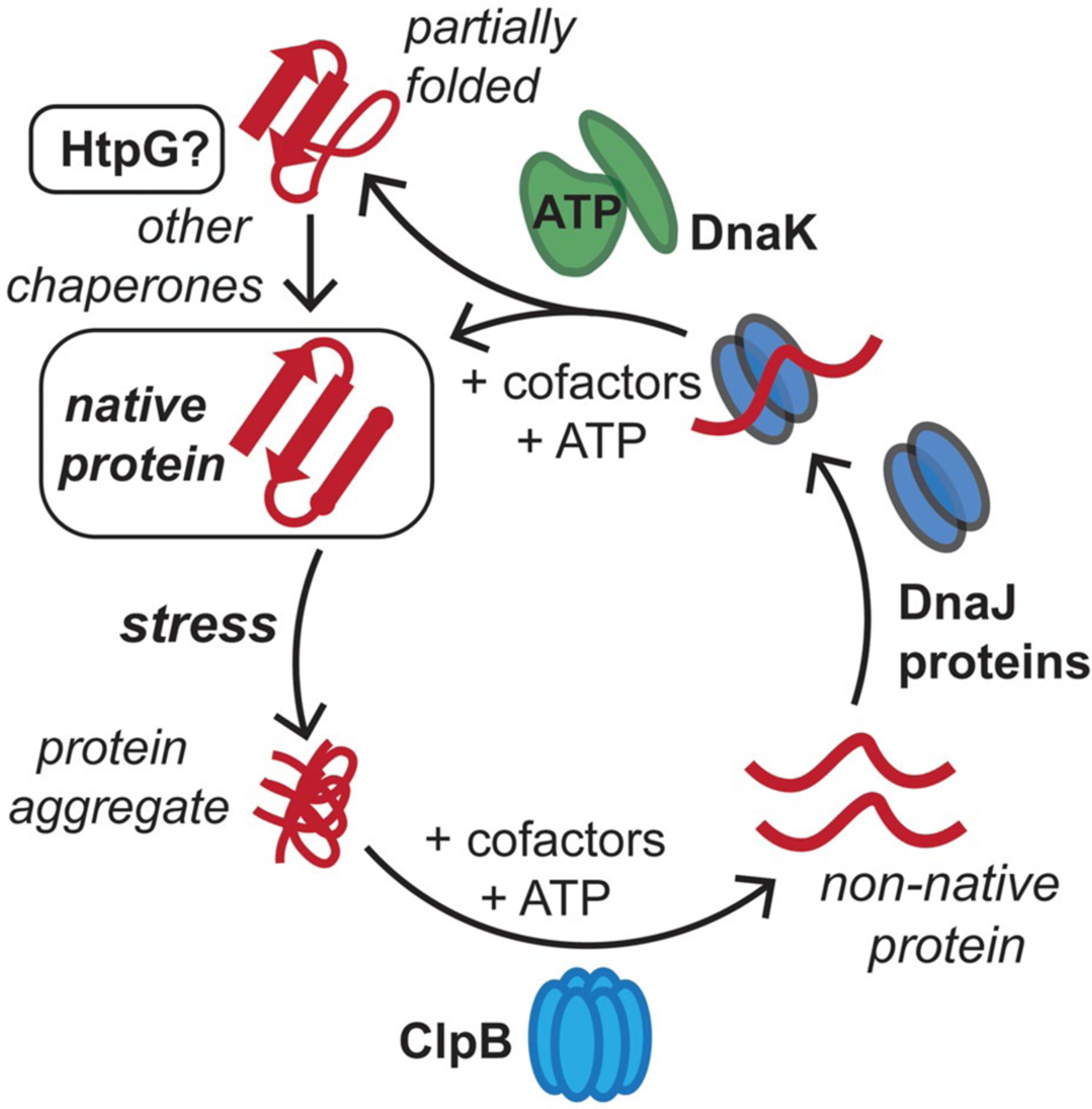
Conserved mycobacterial protein chaperones reactivate aggregated proteins that result from stresses in the host. M. tuberculosis (Mtb) cells are exposed to reactive oxygen and nitrogen species, along with antibiotics, in the host, which can lead to protein aggregation. The conserved bichaperone system consisting of ClpB and DnaK, along with cofactor proteins including DnaJs and GrpE, can help reactivate these proteins to reform native protein folds using ATP as an energy source. Here, we assess whether Mtb HtpG assists the ClpB-DnaK chaperone network in protein folding and homeostasis in response to stress. This figure has been modified from (Lupoli et al., 2018)
