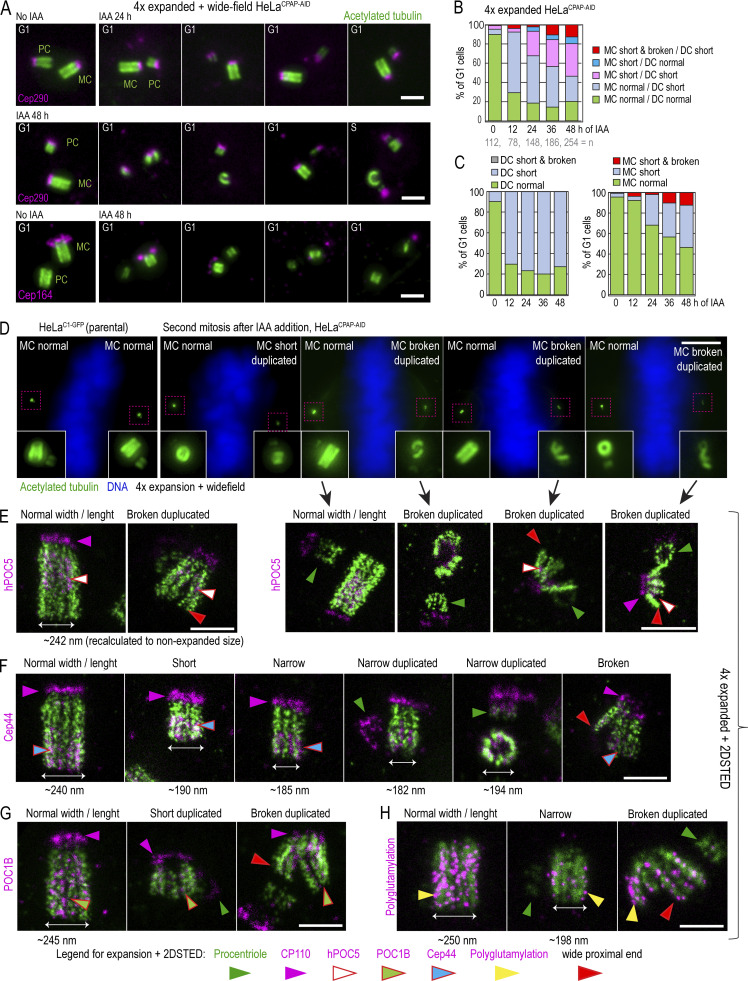
Figure 7.
CPAP removal leads to the assembly of structurally defective centrioles that destabilize on proximal ends. Cells were synchronized by mitotic shake-off (t = 0 h) and treated with IAA 6 h after shake-off. Cells were fixed when noted, expanded approximately fourfold, immunolabeled using acetylated tubulin to label centriole MT walls (green) and various centrosomal proteins (magenta), and imaged. (A) Examples of centriole configurations in control and IAA-treated cells. (B and C) Characterization of the phenotypes of MC-DC pairs in G1 cells at indicated times. Phenotypes of MCs and DCs are additionally shown individually in C. (D) Wide-field images of mitotic cells after 48 h of IAA treatment containing one normal-looking and one aberrant mother centriole. 2DSTED high-resolution images of four centrioles indicated by arrows are shown below. (E–H) Examples of expanded centrioles after 36–48 h of IAA treatment, labeled with acetylated tubulin and colabeled with centriole cap protein CP110, centriole distal protein hPOC5 (E), centriole proximal protein Cep44 (F), centriole luminal protein POC1B (G), and polyglutamylated tubulin (H). The panels illustrate mother centrioles that lost cohesion on proximal ends (red arrows) and that have shorter and narrower acetylated tubulin signal than controls. M, mitosis. Scale bars: 2 µm (A); 20 µm (wide-field in D); 2 µm (2DSTED in D); 1 µm (E–H).