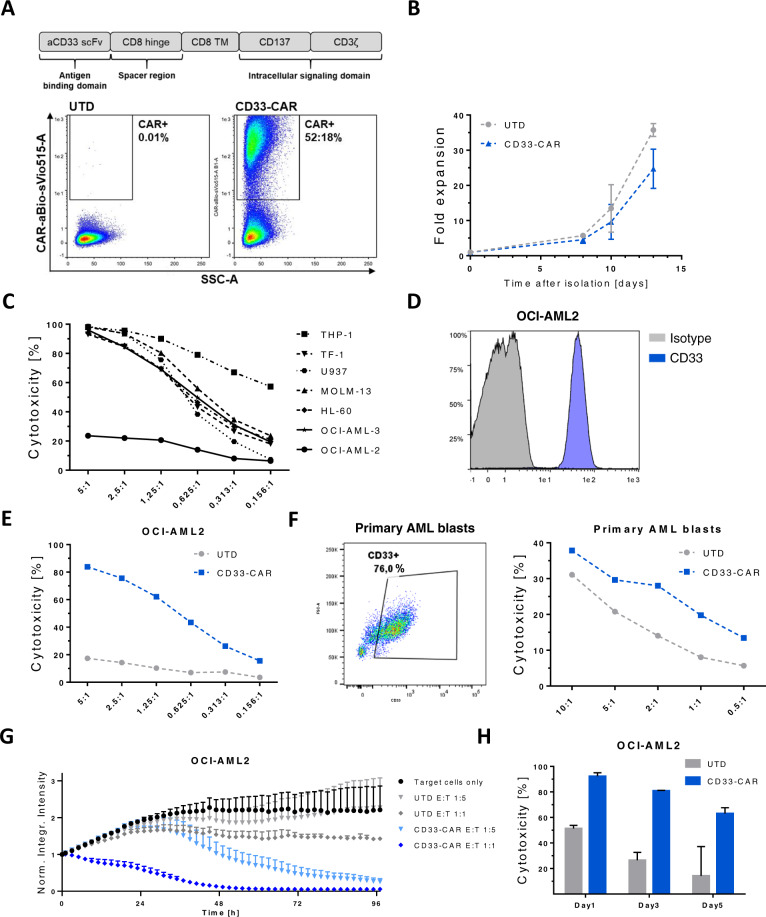
Fig. 1. CD33-CAR-NK cells display robust in vitro effector function against CD33-positive AML cells that are partially resistant to natural cytotoxicity.
A Schematic representation and surface expression of the CD33-directed second-generation CAR used in this study. Expression was analyzed by flow cytometry 12 days after transgene transfer into primary NK cells. B Time-lapsed expansion of CAR-transduced (CD33-CAR) and untransduced (UTD)-NK cells in the presence of IL-2 (500 IU/mL) and IL-15 (140 IU/mL) (n = 5). C Expanded NK cells show high cytotoxic activity against various AML cell lines except OCI-AML2. On day 14 of expansion, NK cells were co-incubated with various AML target cells at indicated E:T-ratios. After 24 h, the fraction of viable target cells was quantified by flow cytometry. Data shown are representative of results from two independent experiments. D The AML cell line OCI-AML2 displays high CD33 surface expression. E, F NK cells equipped with a CD33-CAR become highly cytotoxic against OCI-AML2 and CD33-positive primary AML cells. Cells were co-cultivated for 4 h and the viability of target cells was quantitated by flow cytometry. Two representative experiments are shown. G Dynamic monitoring of CAR-NK cell-mediated cytotoxicity. On day 12 after transduction, CAR-NK cells were co-cultured with (GFP+) OCI-AML2 cells and fluorescence emission was measured in the IncuCyte S3 imaging platform over 4 days. Shown is one representative from three separate experiments with a total of 5 donors. H Repetitive tumor-challenge assay revealed superior serial killing capacity of CD33-CAR-NK cells compared to UTD-NK cells. Expanded NK cells at day 12 post transduction were co-cultured with OCI-AML2 cells at an E:T-ratio of 1:1 and re-challenged with AML cells every other day. Shown is one representative experiment with a total of two donors. All graphs show mean of replicated ± SD.