Figure 2.
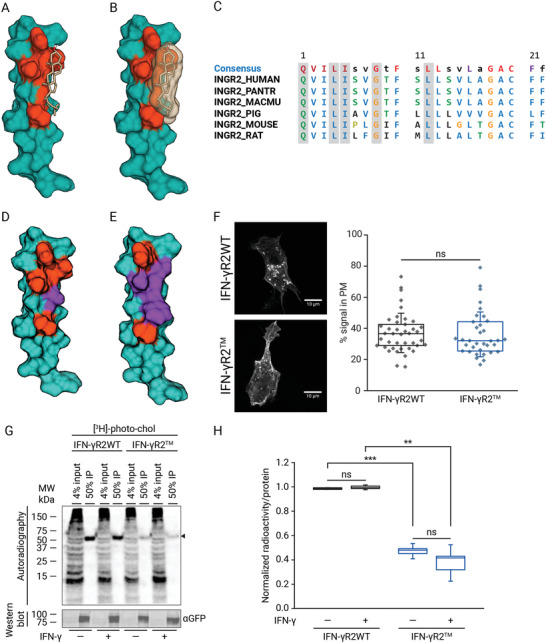
A new conserved chol‐binding domain within the IFN‐γR2‐TMD. A,B) Molecular docking prediction of the chol‐IFN‐γR2TMD interaction. In (A) amino acids forming the binding groove are highlighted in the 3D minimal energy structure of the IFN‐γRTMD. Dark Green, IFN‐γR2TMD + 3 N‐terminal juxtamembrane residues; orange, chol‐binding pocket; light‐brown, chol. B) Chol space‐filling occupancy within the binding domain highlighted in orange as described in (A) in a 3D minimal energy structure of the IFN‐γR2TMD. C) Conservation analysis of the chol‐binding domain across IFN‐γR2TMD in different species. The conserved residues are shaded in red and amino acids forming the chol‐binding pocket are boxed in blue. D,E) Energy‐minimized structure of IFN‐γR2TMD mutants. D) IFN‐γR2G254S energy‐minimized structure. Residues forming the chol‐binding pocket are depicted in orange and the G254S mutation is highlighted in purple. E) Energy‐minimized structure of IFN‐γRTM mutant. Residues forming the chol‐binding domain are highlighted in orange; L250T, I25T, and G254F mutations are depicted in purple. F) Fluorescence microscopy images of transiently expressed full‐length IFN‐γR2WT‐GFP and IFN‐γR2TM‐GFP proteins localization in HAP1IFN‐γR2KO cells (left panel) and PM localization of the wild‐type and mutant receptor (right panel) (n = 3 independent experiments). Scale bar = 10 µm. G) In vivo binding of the bifunctional chol probe to full‐length IFN‐γR2WT and IFN‐γR2TM proteins in non‐treated versus IFN‐γ stimulated HAP1IFN‐γR2KO cells. Arrow, expected protein size (n = 3 independent experiments). H) Quantification of immunoprecipitated radioactivity/protein for chol binding (data are the mean ± SD; n = 3 independent experiments). The line on each of the boxes represents the median for that particular data set). Statistical significances were determined with one‐way ANOVA Bonferroni's multiple comparison test (***p < 0.001; **p < 0.01; ns: not significant).
