Abstract
Background:
A quality improvement study done at the Medical College of Wisconsin between 2014 and 2016 demonstrated that, at baseline, sequential compression devices (SCD) were ordered for 46.0% of admitted antepartum women. In response, provider education and a pre-checked SCD order in the electronic antepartum admission order set were implemented.
Objective:
To examine the effect of these interventions on SCD compliance during antepartum admissions.
Study Design:
This was a prospective observational study of antepartum women admitted for non-delivery indication for more than 24 hours, from June 2017 through March 2018, in a single tertiary center. The study was conducted a year after provider education and implementation of a pre-checked order for SCD in the electronic anterpartum admission order set. Women with an active venous thromboembolism (VTE) and those already receiving pharmacologic thromboprophylaxis were excluded. The primary outcome was the rate of SCD compliance, assessed both among obstetric providers and patients. SCD compliance for providers was defined as SCD order present in patient’s electronic medical record and documenting the presence of SCD in patient’s room. SCD compliance for patients was defined as documentation that the patient was wearing SCD that were turn on while in bed during morning study rounds.
Results:
During the study period a total of 182 rounding encounters were documented for 76 women. SCD was ordered in 77.6% (59/76) of the admissions. Out of the 59 electronic orders for SCD, 45 orders (h 76.3%) were placed on hospital day 1. ( and 42 orders had confirmation of SCD present in the room (71.2%). SCD were in active use in 45.2% (19/42) of these women. When evaluating the daily course of the hospitalization (n=182), SCD were ordered in 86.8% (158/182) of the encounters and present in the room in 72.2% (114/158) of the daily encounters. After excluding 10 women who were ambulatory at the time of rounding (n=104), SCD were observed being used in 31.7% (33/104) of the non-ambulatory women encounters with SCD ordered and present in the room.
Conclusion:
A pre-checked antepartum order set for SCD increased the rate of provider compliance with SCD. However, this increase did not result in high patient compliance with SCD among antepartum women requiring admission for longer than 24-hours.
Keywords: sequential compression device, pregnancy, antepartum, admission, compliance
Venous thromboembolic event is an important preventable cause of morbidity and mortality in hospitalized patients.1–5 One of the prophylactic inpatient standards for venous thromboembolism (VTE) is intermittent pneumatic compression devices, also known as sequential compression devices (SCD).6–7 SCD have been shown to significantly reduce VTE in hospitalized patients.8–10 However, previous studies demonstrated that patient compliance with SCD is low among patients admitted both to the medical and to the surgical wards.5,11–12 Furthermore, compliance decreased as the length of the hospital stay increased.13–15 Compliance is highly important to the prophylactic effects of SCD since their protective effects are reversed after only 10 minutes from the time of SCD removal.16
Pregnancy and postpartum status are independent risk factors for VTE, one of the main causes of maternal death in the United States.17–18. Analysis of a national U.K.database of more than 200,000 women hospitalized during pregnancy for non-delivery indications showed that the risk of VTE increased by 18-fold during anteapartum admission compared to the time outside the hospital.19 SCD have been recently recommended as mechanical thromboprophylaxis for women hospitalized during pregnancy who are at high risk for childbirth or bleeding, instead of pharmacologic prophylaxis.20–21. SCD are also recommended for all women undergoing cesarean delivery who are not already receiving pharmacologic thromboprophylaxis.22 While the use of SCD during antepartum admissions is becoming increasingly common,23 studies assessing SCD utilization and compliance in this population are still lacking.
After publication of “National Partnership for Maternal Safety: Consensus Bundle on Venous Thromboembolism”24 bringing awareness to the subject of VTE prevention in pregnancy and postpartum, a quality improvement project was conducted at Froedtert Hospital at the Medical College of Wisconsin to assess the rate of obstetricians ordering SCD for antepartum admission not leading to delivery between 2014 and 2016. The quality improvement project found that during the baseline year 2014, the rate of SCD order for VTE prophylaxis by hospital day 2 was 46.0%. In response, an intervention of automatic pre-checked order for SCD was implemented in the antepartum admission order set. In addition, physicians and nurses underwent brief education sessions on VTE prophylaxis guidelines. These guidelines and reminders to order SCD were also displayed at all work stations on labor and delivery and antepartum units. By June 2016, 3 months after the completion of QI project, these interventions increased the rate at which orders for SCDs were placed to 74.0%. The objective of this study was to assess whether this intervention led to an increase in SCD compliance during antepartum admission for undelivered women.
Materials and methods
This was a prospective observational study of women admitted to Froedtert Hospital from June 2017 through March 2018 during pregnancy for non-delivery indication. Froedtert Hospital is a 550-bed, quaternary care hospital affiliated with the Medical College of Wisconsin, at which approximately 2,333 deliveries were performed during the study period. All care is documented within an electronic medical record (EMR) Epic (Verona, Wisconsin). The study was certified as a Quality Improvement (QI) Study by the Institutional Review Board at the Medical College of Wisconsin and did not require patient consent. This study period was chosen as it was 1 year after the completion of provider education and implementation of pre-checked SCD order in the antepartum admission order set. The current study included pregnant women ≥18 years of age requiring inpatient antepartum hospitalization longer than 24 hours for non-delivery indication. Women with an active VTE and those already receiving pharmacological anticoagulation were excluded. For women with multiple admissions over the study period, only the first admission was included. In addition, postpartum women were excluded as our goal was to assess SCD compliance during the antepartum period only.
The primary outcome of this study was rates of SCD compliance, assessed both among obstetric providers and pregnant women. SCD compliance for providers was defined as placing the SCD order and documenting the presence of SCD in patient room. SCD compliance for pregnant women requiring antepartum admission was assessed by direct observation documenting that the patient was wearing SCD while in bed. In addition, we planned to compare provider SCD compliance by comparing the rates of SCD orders placed before and after the QI intervention.
During daily morning rounds, Monday through Sunday, between 7am-10am, study investigators reviewed the EMR and went to patient rooms of women meeting the inclusion criteria in order to assess the following three items: 1) whether the SCD order was placed (through EMR patient chart review); 2) whether the SCD were in the room; and 3) whether the patient was wearing the device while in bed. Obstetric provider compliance was assessed by the first two items. Patient’s compliance was assessed by the third item. The assessments started on hospital day 2, after confirmation that the patient required admission longer than 24 hours. The investigators did not expect the patient to start wearing SCD on hospital day 1 as day one included triage and assessment of the need for admission versus discharge; thus the treatment team was not expected to order SCD on hospital day 1. However, it was expected that the order for SCD on the antepartum order set admission would be checked on hospital day 1, after the decision regarding admission had been made. SCD must have been on the patient’s lower extremities and the machine turned on in order for the patient to be deemed compliant. It was also noted if the patient was ambulating during rounding as SCD prophylaxis is only required when patients are in bed. SCD compliance was assessed both by admission data as an entire hospitalization as well as by encounter data which examined SCD compliance on each hospital day of each admission.
The following data was abstracted from the EMR: maternal age, maternal race and ethnicity, maternal body mass index (BMI), parity, hospital day during each patient encounter, primary admission reason, gestational age during admission, preexisting medical conditions and length of hospital stay.
Descriptive statistics and a univaraible comparison using chi square test were performed using Stata version 14.2 (StataCorp College Station, TX). To prevent the Hawthorne effect, defined as a change in behavior among individuals who are aware of being observed, not every hospital day was captured for each admission included in our study. If the patient was admitted for longer than 5 days, the study team did not assess SCD compliance for day 6th and 7th of the admission and resumed study rounding on hospital day 8th.
Results
During the study period, a total of 76 admissions and 182 encounters were eligible and observed. Maternal characteristics are shown in Table 1. The most common indication for hospitalization was preeclampsia (23.7%). The mean BMI of antepartum women was 32.5 ± 8.27 kg/m2. The mean length of hospital admission was 7.41 ± 6.71 days. There were no episodes of VTE among antepartum admissions during the study period.
Table 1.
Maternal characteristics and primary indication for antepartum admission
| Maternal Characteristics | Value (n=76) | Primary indication for admission | Value (n=76) |
|---|---|---|---|
|
| |||
| Age (years) | Preeclampsia | 18 (23.7) | |
| Mean (SD) | 28.5 ± 5.45 | Preterm Labor | 14 (18.4) |
| Median (IQR) | 28 (18, 39) | Cervical Insufficiency/Dilation | 10 (13.2) |
| Race | PPROM | 6 (7.9) | |
| Non-Hispanic White | 32 (42.1) | Rule out Preeclampsia | 4 (5.3) |
| Non-Hispanic Black | 33 (43.4) | FGR Monitoring | 4 (5.3) |
| Hispanic | 6 (7.9) | Pyelonephritis | 3 (4.0) |
| Other | 4 (5.3) | Bleeding from Placenta Previa | 2 (2.6) |
| Not documented | 1 (1.3) | Poorly controlled DM | 2 (2.6) |
| Length of Hospital Stay | Kidney Stone | 2 (2.6) | |
| Mean (SD) | 7.41 ± 6.71 | Labial Abscess | 1 (1.3) |
| Median (IQR) | 5 (2, 28) | Placental abruption | 1 (1.3) |
| Body Mass Index (kg/m2) | Oligohydramnios | 1 (1.3) | |
| Mean (SD) | 32.5 ± 8.27 | Opioid Withdrawal | 1 (1.3) |
| Median (IQR) | 30.6 (19.8, 49.9) | Category II fetal heart rate | 1 (1.3) |
| Gestational Age at time of admission (wks) | Radio-frequency ablation | 1 (1.3) | |
| Mean (SD) | 29.7 ± 4.85 | Sickle Cell Crisis | 1 (1.3) |
| Median (IQR) | 29.3 (19.3, 37.0) | Fetal SVT | 1 (1.3) |
| Nulliparity | 25 (32.9) | ||
| Diabetes | 11 (14.5) | ||
| Multifetal gestation | 14 (18.4) | ||
| Chronic Hypertension | 12 (15.8) | ||
| Preeclampsia | 24 (31.6) | ||
| Preterm Labor | 16 (21.1) | ||
| Preterm prelabor rupture of membranes | 7 (9.2) | ||
All data presented as mean standard deviation, median and inter-quartile range or N (%)
PPROM, preterm premature rupture of membranes; FGR, fetal growth restriction; DM, diabetes mellitus; SVT, supraventricular tachycardia
A pre-checked SCD order in the antepartum admission order set led to an increase in SCD orders. SCD were ordered in 59 (77.6%) of the total 76 admissions, which was statistically significantly higher than the baseline rate of 46.0% (p=0.003). Of those SCD orders placed, 76.3% (45/59) were ordered on hospital day 1 and an additional 13.6% (8/59) of these orders placed on hospital day 2. The rest were ordered after 2 days of admission (10.1%, 6/59). During morning study rounds for 59 admissions that had EMR orders for SCD, SCD were in patient’s room in 71.2% (42/59). Of these, SCD were observed being actively used in 45.2% (19/42) of patients.
When evaluating all 182 encounters, in 164 of these encounters women were non-ambulatory (i.e. lying in bed). SCD orders were placed in the EMR in 86.8% (158/182) of all encounters. Of those encounters, SCD was present in the room in 72.2% (114/158). Ten of the 114 encounters with SCD in the room demonstrated ambulating women. In the remaining 104 non-ambulatory encounters, SCD were observed being used by the patients in 31.7% (33/104) of encounters.
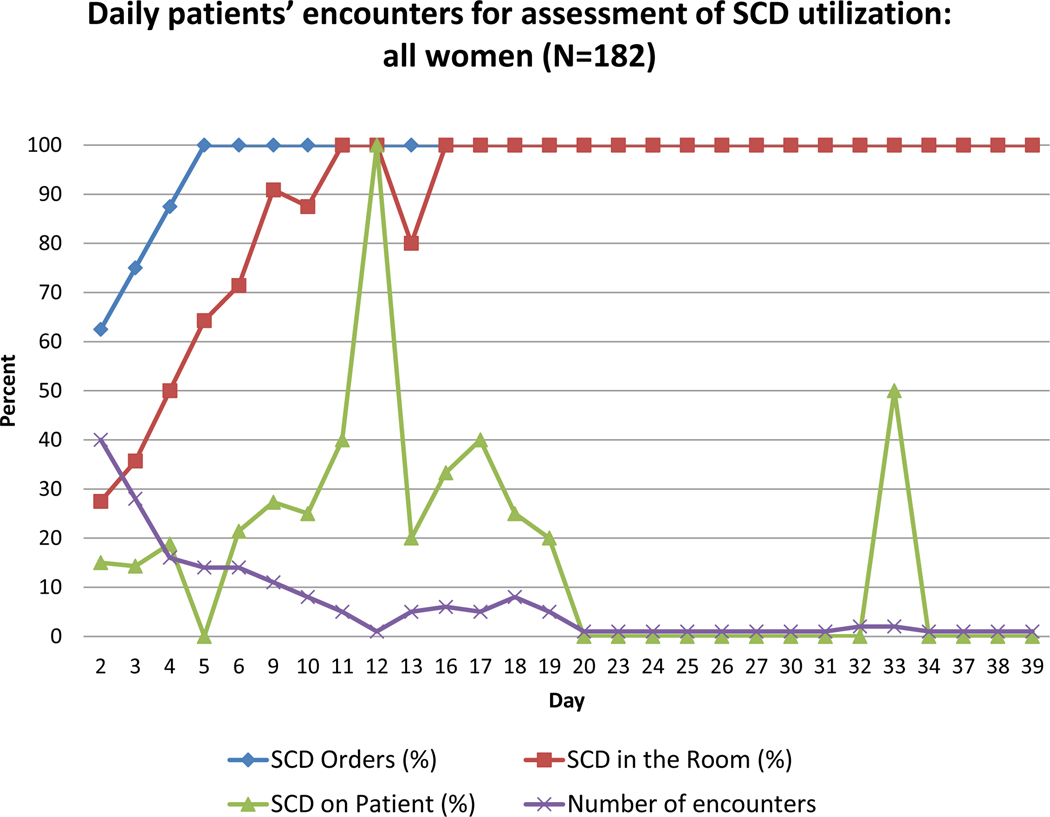
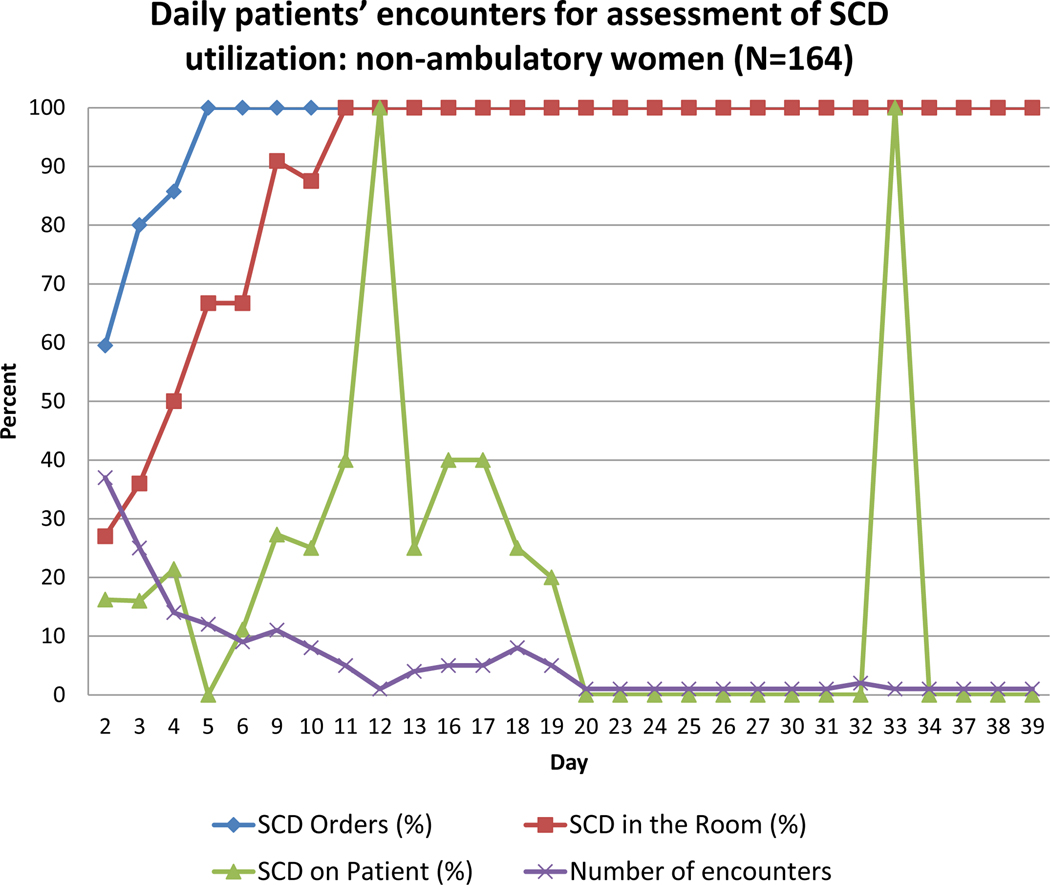
Figures 1 and 2 display the number of daily encounters and percent of SCD orders, SCD in the room, and SCD on the patient being used, for all women (Figure 1) and for non-ambulatory women (Figure 2). The figures demonstrate that SCD were not ordered consistently until hospital day 5. SCD were not present consistently in the room until hospital day 11, which is a 6-day delay between placement of SCD order and delivery of the SCD to patient’s room. Patient SCD compliance rate (i.e., the patient wearing SCD while in bed) showed no clear trend as hospital day number increased and on average was 37.9% by hospital day 20.
Figure 1.
Daily observations for assessment of SCD utilization among all anteparum women (n=182). The purple line demonstrates how many encounters were made on each hospital day. The blue line demonstrates the percent of SCD orders placed on each hospital day. The red line demonstrates percent of SCD present in the room on each hospital day. The green line demonstrates variation in SCD use by patients on each hospital day. The denominator for each day is the number of encounters.
Figure 2.
Daily observations for assessment of SCD utilization among non-ambulatory antepartum women (n=164). The purple line demonstrates how many encounters were made on each hospital day. The blue line demonstrates the percent of SCD orders placed on each hospital day. The red line demonstrates percent of SCD present in the room on each hospital day. The green line demonstrates variation in SCD use by patients on each hospital day. The denominator for each day is the number of encounters.
Discussion
Principal Findings
The objective of this study was to evaluate SCD compliance both among obstetric providers and pregnant women requiring antepartum admission. We found that having a pre-checked antepartum admission SCD order significantly increased the number of provider compliance with SCD. However, this increase in SCD orders did not result in high patient compliance with SCD use.
Results
Overall SCD compliance among women using the ordered and available device was 45.2% of all admissions and 31.7% of daily encounters in which patients were non-ambulatory and SCD were in the room. Among all 182 encounters, women were observed ambulating (or not in their room) in only 18 (9.9%), which suggests this did not primarily contribute to low compliance. In contrast, we were pleased and surprised to note that the rate of SCD ordering 1 year after the completion of provider education and implementation of the pre-checked SCD order on hospital day one was 76.3%, which was significantly higher than the baseline rate of 46.0%, prior to the QI intervention. The fact that this compliance is less than 100% could possibly be explained by providers either using their custom-order set or remaining unconvinced of the importance of VTE prophylaxis, due to the overall low incidence of VTE during pregnancy.
Previous studies assessing utilization of SCD and patient compliance in non-pregnant populations reported better compliance rates with SCD, ranging from 52.4% to 79.5%. 13–14, 25–26. A 2009 study by Bockheim et al compared SCD compliance between surgical ICU and non ICU patients and showed that mean compliance over repeat encounters in ICU patients was 82%, and higher than in non-ICU patients (62%).14 Comparatively, a study done on post-operative urology patients showed 78.6% compliance with SCD usage.13 Another study of non-ambulatory trauma patients observed 53% compliance with SCD.27
Studies assessing SCD compliance among pregnant antepartum admissions are lacking as until recently there were no specific recommendations regarding SCD use in pregnant women with non-delivery hospitalizations. Mardy et al performed a retrospective chart review to analyze mechanical and pharmacologic thromboprophylaxis in this population.23 They found that between 2006 and 2015 the use of thromboembolism prophylaxis increased by 100% and among antepartum admissions, 87.2% of the prophylaxis was mechanical. However, as this was retrospective chart review, there was no information about how many of these women were actually using the device. We were not able to find any additional studies investigating SCD compliance during antepartum period. Two studies reported compliance rates with SCD use after cesarean delivery.25–26 Brady et al demonstrated compliance of 52.4% with SCD use after cesarean delivery,25 while Palmerola et al reported compliance of 79.5% after cesarean delivery.26 Their compliance rate was higher likely due to the fact that they only assessed SCD compliance on post operative day 1. These studies identified the following barriers to optimal SCD compliance: patient discomfort, device not being in the room, SCD not being reinstated after patients returned to bed, patients planning to ambulate and patients being told by nursing staff that they no longer needed SCD.
Research Implications
Future direction of this study will be interviewing noncompliant providers and pregnant women requiring antepartum admission to explore barriers to SCD compliance. In addition, we plan to conduct a QI project to understand and breech the gap between universal pre-checked SCD order and sub-optimal rates of SCD actually being ordered, as well as the 6-day delay on SCD delivery to patient room. The latter findings point towards the need to enhance education regarding the importance of VTE prophylaxis among the nursing staff.
Strengths and Limitations
In our investigation we did not interview women about challenges with SCD compliance and thus we are unable to explain why the rates of SCD compliance were low. A recent study from the U.K. surveyed women regarding SCD use in the postpartum period.27 The authors identified the following barriers to SCD use: loudness and keeping the mother and the baby awake. One third felt that they restrict mobility.27 The data on antepartum barriers to SCD use as reported by women is still unknown. An additional limitation of our study is that we did not plan to assess SCD efficacy, but did note that we had no episodes of VTE during the study period. In light of the small sample size and relatively rare incidence of VTE in pregnancy, 1–2 per 1000,20 this outcome was not part of our study design. Finally, we did not compare patient compliance with SCD prior to institutional implementation of universal antepartum pre-checked SCD order set to the current results. Not collecting this data is consistent with best practices for conducting a QI project where only the data necessary to demonstrate the need for improvement is collected. The baseline phase of QI intervention was focused on the providers’ ordering as the patient will not have the chance to comply without an order for SCD.
In contrast, the strengths of this study include its prospective design which allowed for accurate information on compliance without the pitfalls of retrospective chart reviews. Additionally, its focus on antepartum women, an understudied population in the field of mechanical thromboprophylaxis, is unique. This population is particularly susceptible to VTE as they are in late pregnancy and spending a large amount of time in bed. Another strength of this study is ability to report the ambulatory status of each woman and exclude women already receiving pharmacologic anticoagulation, thus targeting the population that is at higher risk and is in need for mechanical thromboprophylaxis. We also attempted to minimize change in patient behavior that can result from taking part in a study-known at the Hawthorne effect-by skipping two days of SCD compliance study rounds. Since there was no trend of an increase in patient SCD use among longer admissions, we don’t believe that there was a Hawthorne effect in our study.
Conclusion
In conclusion, our study showed that a pre-checked antepartum order set for SCD increased the rate of SCD orders however did not lead to a high patient compliance with SCD among pregnant women requiring antepartum admission for more than 24 hours.
Acknowledgments
Financial disclosures: This publication was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number UL1TR001436. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH
Footnotes
Condensation: A pre-checked order for SCD did not lead to high SCD compliance among admitted antepartum women
Each author has indicated that he/she has met the journal’s requirements for authorship.
The authors did not report any potential conflict of interest.
References:
- 1.Heit JA. Venous thromboembolism: disease burden, outcomes and risk factors. J Thromb Haemost 2005;3:1611–7. [DOI] [PubMed] [Google Scholar]
- 2.Beckman MG, Hooper C, Critchley SE, Ortel TL. Venous thromboembolism: a public health concern. Am J Prev Med 2010;38:S495–501. [DOI] [PubMed] [Google Scholar]
- 3.Deitelzweig SB, Johnson BH, Lin J, Schulman KL. Prevalence of clinical venous thromboembolism in the USA: current trends and future projections. Am J Hematol. 2011;86:217–20. [DOI] [PubMed] [Google Scholar]
- 4.Office of the Surgeon General (US); National Heart, Lung, and Blood Institute (US). The Surgeon General’s Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism. Rockville (MD): Office of the Surgeon General (US); 2008. Available from: https://www.ncbi.nlm.nih.gov/books/NBK44178/ [PubMed] [Google Scholar]
- 5.Spirk D, Nendaz M, Aujesky D, Hayoz D, Beer JH, Husmann M, et al. Predictors of thromboprophylaxis in hospitalised medical patients. Explicit ASsessment of Thromboembolic RIsk and Prophylaxis for Medical PATients in SwitzErland (ESTIMATE). Thromb Haemost. 2015;113:1127–34. [DOI] [PubMed] [Google Scholar]
- 6.Roberts VC, Sabri S, Beeley AH, Cotton LT. The effect of intermittently applied external pressure on the haemodynamics of the lower limb in man. Br J Surg 1972;59:223–6. [DOI] [PubMed] [Google Scholar]
- 7.Comerota AJ, Chouhan V, Harada RN, Sun L, Hosking J, Veermansunemi R, et al. The fibrinolytic effects of intermittent pneumatic compression: mechanism of enhanced fibrinolysis. Ann Surg 1997;226:306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ho KM, Tan JA. Stratified meta-analysis of intermittent pneumatic compression of the lower limbs to prevent venous thromboembolism in hospitalized patients. Circulation 2013;128:1003–20. [DOI] [PubMed] [Google Scholar]
- 9.Sachdeva A, Dalton M, Amaragiri SV, Lees T. Elastic compression stockings for prevention of deep vein thrombosis. Cochrane Database Syst Rev 2010;CD001484. [DOI] [PubMed] [Google Scholar]
- 10.Feng JP, Xiong YT, Fan ZQ, Yan LJ, Wang JY, Gu ZJ. Efficacy of intermittent pneumatic compression for venous thromboembolism prophylaxis in patients undergoing gynecologic surgery: A systematic review and meta-analysis. Oncotarget 2017;8:20371–20379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Comerota AJ, Katz ML, White JV. Why does prophylaxis with external pneumatic compression for deep vein thrombosis fail? Am J Surg 1992;164:265–8. [DOI] [PubMed] [Google Scholar]
- 12.Apenteng PN, Fitzmaurice D, Litchfield I, Harrison S, Heneghan C, Ward A, et al. Patients’ perceptions and experiences of the prevention of hospital-acquired thrombosis: a qualitative study. BMJ Open 2016;6:e013839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ritsema DF, Watson JM, Stiteler AP, Nguyen MM. Sequential compression devices in postoperative urologic patients: an observational trial and survey study on the influence of patient and hospital factors on compliance. BMC Urol 2013;13:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bockheim HM, Mcallen KJ, Baker R, Barletta JF. Mechanical prophylaxis to prevent venous thromboembolism in surgical patients: a prospective trial evaluating compliance. J Crit Care. 2009;24:192–6. [DOI] [PubMed] [Google Scholar]
- 15.Obi AT, Alvarez R, Reames BN, et al. A prospective evaluation of standard versus battery-powered sequential compression devices in postsurgical patients. Am J Surg. 2015;209:675–81. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs DG, Piotrowski JJ, Hoppensteadt DA, Salvator AE, Fareed J. Hemodynamic and fibrinolytic consequences of intermittent pneumatic compression: preliminary results. J Trauma. 1996;40:710–16. [DOI] [PubMed] [Google Scholar]
- 17.Heit JA, Kobbervig CE, James AH, Petterson TM, Bailey KR, Melton LJ. Trends in the incidence of venous thromboembolism during pregnancy or postpartum: a 30-year population-based study. Ann Intern Med. 2005;143:697–706. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. Pregnancy Mortality Surveillance System. Available at: https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pregnancy-mortality-surveillance-system.htm. Retrieved November 1, 2018.
- 19.Sultan AA, West J, Tata LJ, Fleming KM, Nelson-piercy C, Grainge MJ. Risk of first venous thromboembolism in and around pregnancy: a population-based cohort study. Br J Haematol 2012;156:366–73. [DOI] [PubMed] [Google Scholar]
- 20.Dʼalton ME, Friedman AM, Smiley RM, Montgomery DM, Paidas MJ, D’Oria R, et al. National Partnership for Maternal Safety: Consensus Bundle on Venous Thromboembolism. Obstet Gynecol. 2016;128:688–98. [DOI] [PubMed] [Google Scholar]
- 21.Sibai BM, Rouse DJ. Pharmacologic Thromboprophylaxis in Obstetrics: Broader Use Demands Better Data. Obstet Gynecol 2016;128:681–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.American College of Obstetricians and Gynecologists. Practice Bulletin No. 196: Thromboembolism in Pregnancy. Obstet Gynecol 2018;132:e1–e17. [DOI] [PubMed] [Google Scholar]
- 23.Mardy AH, Siddiq Z, Ananth CV, Wright JD, DʼAlton ME, Friedman AM. Venous Thromboembolism Prophylaxis During Antepartum Admissions and Postpartum Readmissions. Obstet Gynecol 2017;130:270–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.“Venous Thromboembolism:Risk Assessment and Prophylaxis”. ACOG Safe Motherhood Initiative. June 2014. https://www.acog.org/About-ACOG/ACOG-Districts/District-II/SMI-Venous-Thromboembolism
- 25.Brady MA, Carroll AW, Cheang KI, Straight C, Chelmow D. Sequential compression device compliance in postoperative obstetrics and gynecology patients. Obstet Gynecol 2015;125:19–25. [DOI] [PubMed] [Google Scholar]
- 26.Palmerola KL, Brock CO, D’alton ME, Friedman AM. Compliance with mechanical venous thromboproembolism prophylaxis after cesarean delivery. J Matern Fetal Neonatal Med 2016;29:3072–5. [DOI] [PubMed] [Google Scholar]
- 27.Guimicheva B, Patel JP, Roberts LN, Subramanian D, Arya R. Women’s views, adherence and experience with postnatal thromboprophylaxis. Thromb Res. 2019;173:85–90. [DOI] [PubMed] [Google Scholar]