Fig. 1. Deep dermal ENFs activate Engrailed-1 and contribute to postnatal scar collagen deposition.
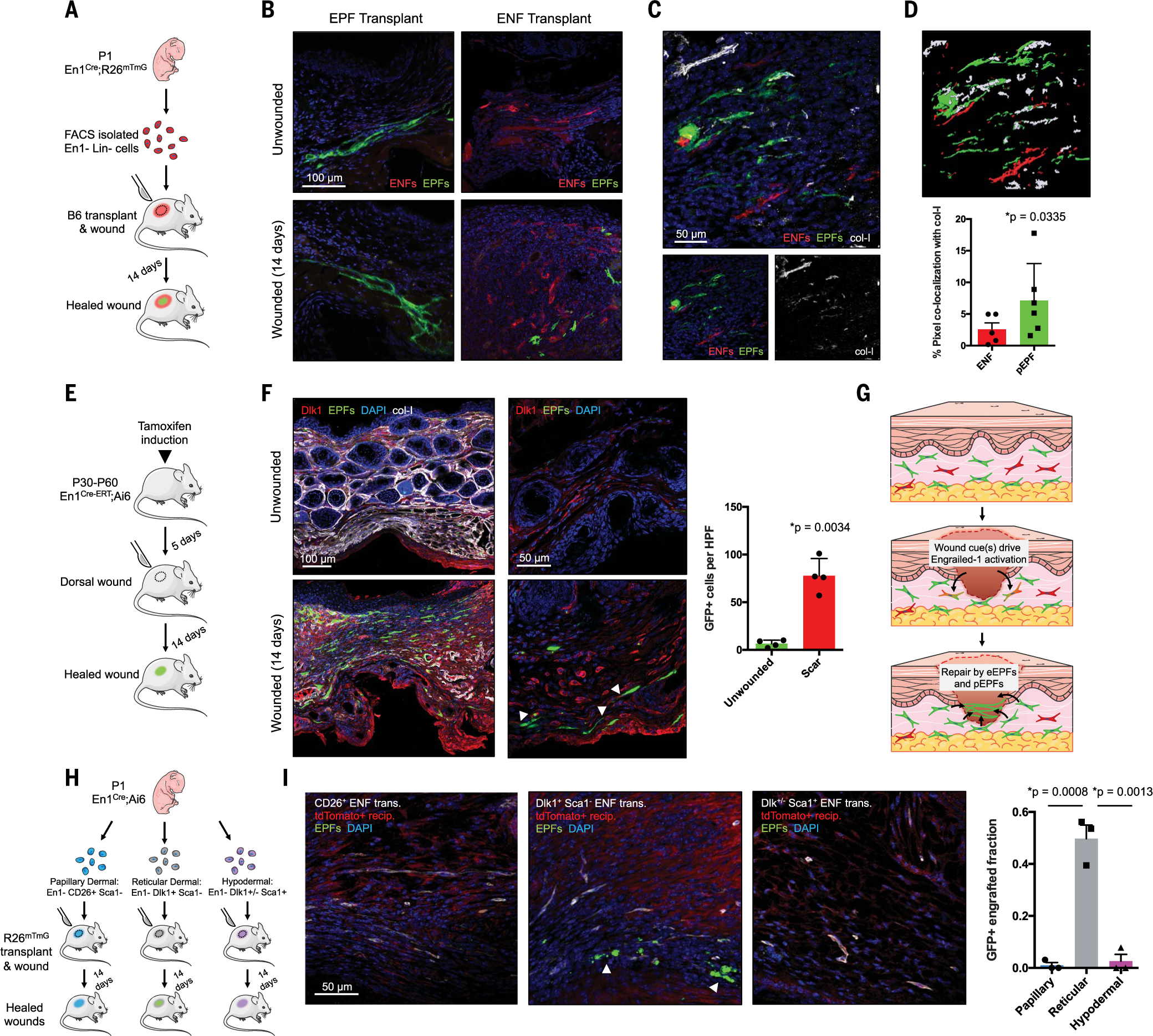
(A) Schematic depicting cell transplantation, engraftment, and wounding experiments. (B) Confocal imaging of transplanted En1-positive fibroblasts (EPFs) and En1-negative fibroblasts (ENFs) before and after wounding [includes 4′,6-diamidino-2-phenylindole (DAPI, blue)]. (C) ENF transplantation and wounding, with postnatal EPFs (pEPFs, green) derived from ENF-to-EPF conversion; immunostaining for type I collagen (col-I). (D) Top: 3D reconstruction of (C). Bottom: Colocalization between col-I and tdTomato (ENF) or GFP (pEPF) signal. (E) Schematic depicting induction and wounding of En1Cre-ERT;Ai6 mice for temporally defined assessment of En1 activation. (F) Left: Skin and wounds of tamoxifen-induced En1Cre-ERT;Ai6 mice; EPFs (white arrowheads) necessarily arose from En1 expression during healing. Immunostaining for Dlk1, col-I. Right: Quantification of GFP+ cells (pEPFs) in unwounded skin or scars; paired two-tailed t test. (G) Proposed mechanism for postnatal En1 activation. Dermal ENFs (red) exposed to wound-specific cues convert to pEPFs, which, with embryonically derived EPFs (eEPFs), mediate scarring. (H) Schematic depicting ENF subtype isolation, transplantation, and wounding. (I) Left: Papillary (CD26+, left), reticular (Dlk1+Sca1−, center), and hypodermal (Dlk1+/−Sca1+, right) ENFs in wounded tdTomato+ recipient (red); only reticular ENFs become pEPFs (white arrowheads). Right: Quantification of pEPFs in subtype-engrafted wounds.
