Abstract
Erythrocytosis is a well-recognized consequence of exogenous testosterone, however its prevalence and contributions to thrombosis remain unknown in the context of gender-affirming hormonal therapy. We undertook a retrospective study of transgender and non-binary (TGNB) adults receiving exogenous testosterone. In the retrospective sample, 923 transgender individuals receiving testosterone were identified with 519 having documented pre- and post-testosterone hemoglobin and hematocrit (Hgb/Hct). The mean peak Hgb/Hct was 15.7 g/dL, and 47.0%. Mean time-to-peak Hgb/Hct was 31.2 months; 7.8% developed a hemoglobin >17.5 g/dL, whereas 20% developed a hematocrit of >50%. Testosterone dose reduction occurred in 42% of patients with erythrocytosis and 4.8% underwent phlebotomy. Thromboembolic events occurred in 0.9%, of which 80% had developed erythrocytosis by either Hgb or Hct, including two cases each of superficial and calf vein thrombosis as well as one ischemic stroke. We then performed an analysis of 14,294,784 hospitalizations from the 2016–17 US National Inpatient Sample (NIS), which identified 4141 admissions involving transgender individuals. Of those, seven had erythrocytosis with one concurrent venous thromboembolic event. Hematocrit >50% occurs in up to 20% of transgender individuals receiving testosterone. Despite the high incidence of erythrocytosis, thromboembolic events and hospitalizations involving erythrocytosis were uncommon.
Keywords: Thrombosis, Transgender, Gender dysphoria, Testosterone, Erythrocytosis
1. Introduction
Up to 0.6% of the United States population identified as transgender in 2016 [1]. Various forms of gender-affirming exogenous testosterone are utilized by some TGNB individuals undergoing transition. Regardless of the formulation, testosterone causes both desired and undesired biologic effects, including hematologic ones such as secondary erythrocytosis, often requiring management by clinical hematologists [2]. The incidence of testosterone-induced erythrocytosis and associated thromboembolic risks in this population have been an area of ongoing investigation with one large study describing low rates compared to cisgender populations [3]. Despite this, in 2015 the FDA announced mandatory warning labels suggesting an increased risk of thromboembolism with all testosterone prescriptions. Furthermore, the ideal management of erythrocytosis remains unknown, with testosterone dose reduction having the potential to limit the desired gender-affirming effects. The aim of this study is to identify the incidence of erythrocytosis, associated thromboembolic events, and the frequency of various management strategies in a large data set of transgender individuals receiving testosterone and in a national hospitalization dataset.
2. Methods
We performed a retrospective observational study of an inpatient and outpatient registry at a large academic medical center in Portland, Oregon of TGNB over the age of 18 years old receiving exogenous testosterone therapy from June 2017 to June 2020. Patients were identified based on SNOMED CT (Systemized Nomenclature of Medicine – Clinical Terms), “gender dysphoria” or “transgender identity,” along with a coexisting testosterone prescription. Data were extracted by trained personnel directly from the medical record including age, blood counts, ferritin, and testosterone prescription information. Erythrocytosis was defined as a hemoglobin > 17.5 g/dL or hematocrit > 50% (the traditional reference range for cisgender men). Testosterone levels were not examined as they were not routinely drawn in correlation with blood counts. Additionally, the practice of monitoring testosterone levels was not uniform among the various providers at our institution. Medical records were reviewed for any relevant documentation of thromboembolism including pertinent imaging, hospitalization records, and anticoagulation prescriptions. Pre-treatment and peak hemoglobin and hematocrit were compared by paired t-tests performed in GraphPad Prism version 8.0.0. STATA version 12.1 was used for descriptive analysis.
An analysis was then performed utilizing the 2016–2017 United States National Inpatient Sample (NIS), encompassing 14,294,7874 hospitalizations. Admissions were identified and filtered using the relevant 10th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10) diagnosis codes. A complete description of the methods and list of the diagnosis codes is included in the Supplemental data. The analysis was performed using Python v. 2.7.18 with metadata association and parsing made possible by the pip package PyHCUP v. 0.1.6.4.
3. Results and discussion
A total of 923 TGNB individuals receiving testosterone were identified in the academic medical center sample with a mean age of 29 years. Of these, 519 had both pre- and post-testosterone hemoglobin and hematocrit documented. Testosterone cypionate was the predominant formulation administered in 837/923 patients (90%). The mean baseline and peak hemoglobin were 13.5 and 15.7 g/dL (Fig. 1) respectively (mean difference 2.54 g/dL, p < 0.0001). Mean baseline and peak hematocrit were 40.2% and 47.0%, respectively (mean difference 6.84%, p < 0.0001). The mean time-to-peak for hemoglobin and hematocrit was 31.2 months. During this time, 41/519 patients (7.8%) developed a hemoglobin greater than 17.5 g/dL compared to 104/519 patients (20%) who developed a hematocrit over 50%. The majority of those who developed erythrocytosis were receiving injectable formulations of testosterone, while only 21 patients who developed erythrocytosis were receiving transdermal formulations. Ferritin levels were only available in 120 patients with a mean value of 50 ng/mL. In those who developed erythrocytosis, testosterone dose reduction was the most common intervention performed in 42%, 4.8% underwent therapeutic phlebotomy, and no change or observation compromised the rest. Thromboembolic events occurred in 5/519 patients (0.9%) with four (80%) of these patients meeting criteria for erythrocytosis at any time during their care (Table 1). Notably none had erythrocytosis at the time their thromboembolic event was diagnosed. The thromboembolic events included two superficial vein thromboses, two calf vein thromboses, and one ischemic stroke. Analysis of the NIS identified 4141 admissions involving TGNB patients. Of these, seven were recognized to have erythrocytosis with just one having a concurrent venous thromboembolic event.
Fig. 1.
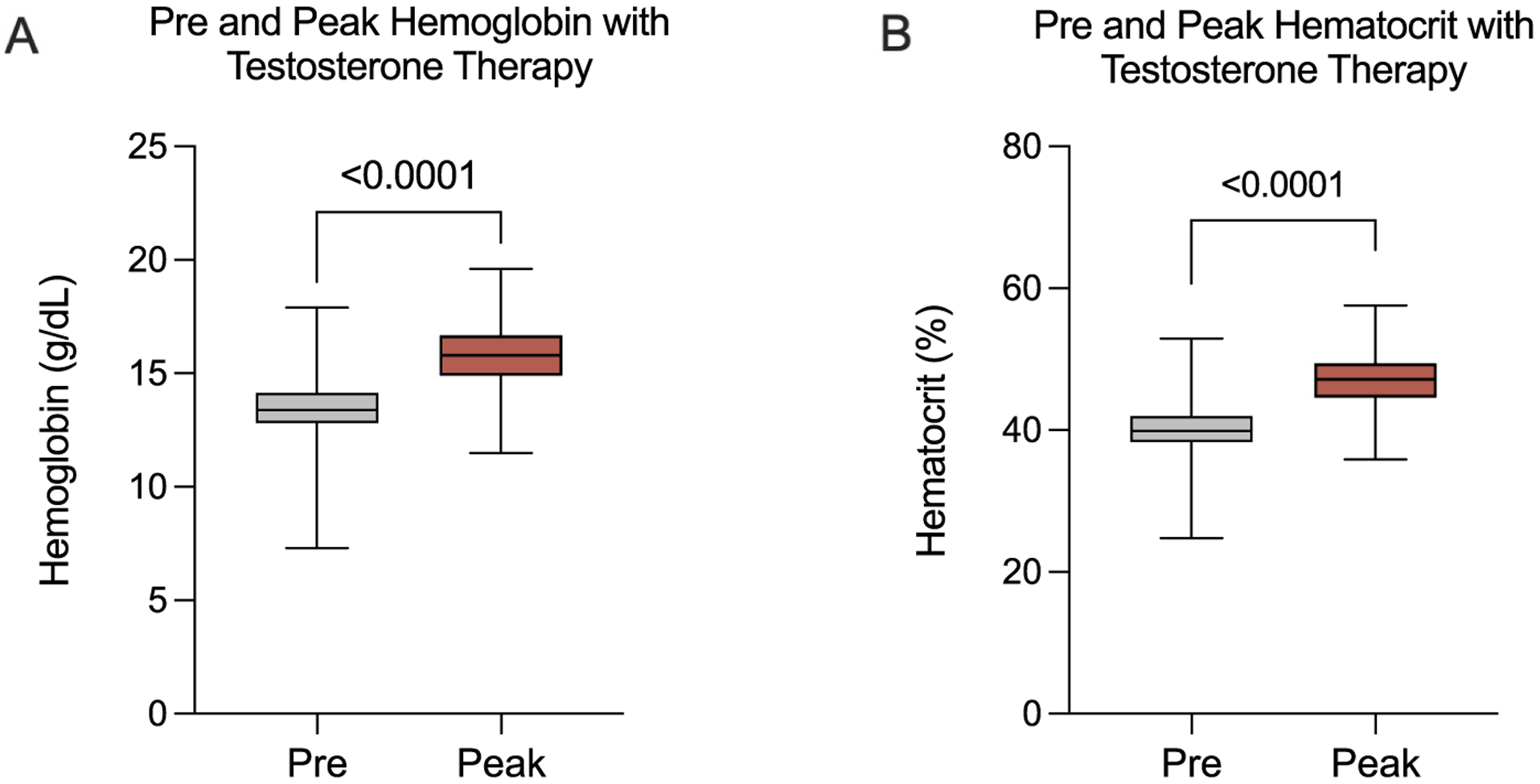
Pre-testosterone and peak hematocrit and hemoglobin during testosterone therapy.
Table 1.
Depicting patients who developed erythrocytosis and thromboembolic events.
| Thromboembolic event | Age (years) | Testosterone | Hgb. (g/dL), Hct. (%) at diagnosis | Months on testosterone at diagnosis | Comorbidities |
|---|---|---|---|---|---|
| Superficial vein thrombosis in greater saphenous vein | 32 | Intramuscular Testosterone Cypionate | 13.2, 38.8 | 49 | Occurred after reconstructive plastic surgery, obesity |
| Non-occlusive thrombus in the anterior tibial vein | 21 | Intramuscular Testosterone Cypionate | 14.5, 43.7 | 36 | Tobacco use, recent leg trauma |
| Ischemic stroke | 35 | Intramuscular Testosterone Cypionate | 14.7, 45.4 | 207 | Dyslipidemia, obesity, tobacco use |
| Non-occlusive R. peroneal & posterior tibial veins | 39 | Intramuscular Testosterone Cypionate | 17.2, 47.7 | 168 | None |
| Superficial thrombus in bilateral saphenous veins | 22 | Intramuscular Testosterone Cypionate | 12.2, 35.2 | 42 | Occurred after reconstructive plastic surgery |
Our analysis found erythrocytosis was common in individuals receiving gender-affirming testosterone, with 1 in 5 patients developing hematocrit >50% after starting treatment. Despite this, thromboembolic events were uncommon (0.9%). Review of a large U.S. hospitalization database reinforced our findings confirming that hospitalizations involving erythrocytosis and thromboembolic events were rare.
Prior studies evaluating testosterone associated erythrocytosis in cisgender populations showed incidences ranging from as low as 0.003% to as high as 66.7% depending on the formulation with short acting intramuscular injections (enanthate and cypionate) having higher rates [4]. The few studies examining testosterone therapy in transgender individuals showed conflicting data with some describing slight hemoglobin and hematocrit increases not significant enough to meet criteria for erythrocytosis [5]. However, recent reports have described erythrocytosis in up to 11% of transgender individuals on testosterone [6]. Higher incidences of erythrocytosis, similar to those seen in our population, have also recently been reported [7]. We attempted to quantify underlying iron deficiency based on ferritin to evaluate whether this decreased the incidence of erythrocytosis. However, ferritin was only available in a small subset of patients.
The relationship between secondary erythrocytosis and thromboembolic risk remains largely unclear in the TGNB population. A prior study of gender-affirming hormone therapy found no thromboembolic events in those receiving testosterone but noted a 12% rate of thromboembolic and cardiovascular events in those receiving estrogen based therapy [8]. The low incidence of thromboembolic events in our study suggests that this may not be a clinically significant factor when pre-scribing testosterone for gender transition. However, it is likely that the risk of thromboembolic events in our study of predominantly young adults is higher when compared to a similar age demographic although it is difficult to draw conclusions from cross-comparison.
Regardless, it is still essential to recognize the development of erythrocytosis to minimize any possible increase in adverse effects. The Clinical Guidelines Subcommittee of the Endocrine Society has published recommendations on testosterone therapy. While extrapolated for hypogonadism, their recommendation is to stop testosterone once hematocrit exceeds 54% and to monitor until it lowers to a “safe level” before resuming at a lower dose [9]. Similarly, the Endocrine Society of Australia recommends reducing the dose or frequency of testosterone although, in rare instances, therapeutic phlebotomy may be necessary [10]. Dose reduction without cessation was the most utilized method in our study. Post-reduction hemoglobin and hematocrits were not examined to determine if the erythrocytosis resolved. It remains unclear how aggressively testosterone-induced erythrocytosis needs to be managed in this population.
There are notable limitations to our study. As this was a retrospective study, there may have been unidentified confounding factors, such as acute illnesses or concurrent conditions that caused patients to develop a secondary erythrocytosis, i.e. tobacco use. Due to the limitations of our data collection, further demographics were not obtained for the entire population, but we were able to obtain them for the thrombosis cohort. Providers may have recommended patients to donate blood rather than undergo scheduled therapeutic phlebotomy, which would not be captured in the medical record. The length of time that patients developed erythrocytosis was not identified, which may have a greater impact on their thrombosis risk than the level of erythrocytosis. In regards to the NIS sample, a majority of the management of TGNB individuals predominantly occurs in the outpatient setting, and thus hospitalization data may underestimate the real number of thrombotic events.
Despite the high incidence of erythrocytosis in our study, it is reassuring to see that thrombosis rates were minimal, although hematologic monitoring and attention to the erythrocytosis still seems warranted. Larger studies at high volume centers are warranted to better define the true thromboembolic risk and ideal management strategy in this population. Further group stratification comparing the incidence of individual types of testosterone therapy and administration routes could determine whether these are associated with a higher risk than others.
Supplementary Material
Key points.
As many as one in five transgender patients receiving exogenous testosterone develop erythrocytosis.
The thromboembolic risk in transgender individuals receiving exogenous testosterone appears to be low.
Funding
This work was supported by grants from the National Institutes of Health, National Heart, Lung, and Blood Institute (R01HL151367, R01HL101972).
Footnotes
CRediT authorship contribution statement
MO, VR, and JJS designed the study. MO, AA, and CK collected the data. MO wrote the manuscript. JS, JJS, BKE, and HDH performed data analysis. JS, HDH, GD, TCLK, CM, and OJTM provided revisions and edits to the manuscript. JJS supervised the study and manuscript writing.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: J. J.S. is a medical consultant for Aronora, Inc. The remaining authors declare no potential conflict of interest.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.thromres.2021.09.005.
References
- [1].Safer JD, Tangpricha V, Care of transgender persons, N. Engl. J. Med 381 (25) (2019) 2451–2460. [DOI] [PubMed] [Google Scholar]
- [2].Shatzel JJ, Connelly KJ, DeLoughery TG, Thrombotic issues in transgender medicine: a review, Am. J. Hematol 92 (2) (Feb 2017) 204–208. [DOI] [PubMed] [Google Scholar]
- [3].Getahun D, Nash R, Flanders WD, et al. , Cross-sex hormones and acute cardiovascular events in transgender persons: a cohort study, Ann. Intern. Med 169 (4) (2018) 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ohlander SJ, Varghese B, Pastuszak AW, Erythrocytosis following testosterone therapy, Sex Med. Rev 6 (1) (Jan 2018) 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chandra P, Basra SS, Chen TC, Tangpricha V, Alterations in lipids and adipocyte hormones in female-to-male transsexuals, Int. J. Endocrinol 2010 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Madsen MC, van Dijk D, Wiepjes CM, Conemans EB, Thijs A, den Heijer M, Erythrocytosis in a large cohort of trans men using testosterone: a long-term follow-up study on prevalence, determinants and exposure years, J. Clin. Endocrinol. Metab 106 (6) (June 2021) 1710–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Defreyne J, Vantomme B, Van Caenegem E, et al. , Prospective evaluation of hematocrit in gender-affirming hormone treatment: results from European network for the investigation of gender incongruence, Andrology 6 (3) (May 2018) 446–454. [DOI] [PubMed] [Google Scholar]
- [8].Wierckx K, Mueller S, Weyers S, et al. , Long-term evaluation of cross-sex hormone treatment in transsexual persons, J. Sex. Med 9 (10) (Oct 2012) 2641–2651. [DOI] [PubMed] [Google Scholar]
- [9].Bhasin S, Brito JP, Cunningham GR, et al. , Testosterone therapy in men with hypogonadism: an Endocrine Society clinical practice guideline, J. Clin. Endocrinol. Metab 103 (5) (2018) 1715–1744. [DOI] [PubMed] [Google Scholar]
- [10].Yeap BB, Grossmann M, McLachlan RI, et al. , Endocrine Society of Australia position statement on male hypogonadism (part 2): treatment and therapeutic considerations, Med. J. Aust 205 (5) (2016) 228–231. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


