Abstract
Hepatic cirrhosis leads to numerous hematologic derangements resulting in a complex and tenuously rebalanced hemostatic milieu. The utility of common hematologic tests including the INR and aPTT in assessing hemostatic and thrombotic risk in patients with cirrhosis is limited, and consensus on transfusion thresholds and proper management of thrombotic complications continues to evolve. This review summarizes the pathophysiology of key derangements of hemostasis including those of platelets, von Willebrand factor, pro- and anticoagulation factors, and fibrin. Additionally, the pathogenesis, consequences, optimal management, and prevention of major thrombotic and bleeding complications in cirrhosis arte discussed.
Keywords: blood coagulation, hemostasis, humans, liver cirrhosis, thrombosis
1 |. INTRODUCTION
Hepatic cirrhosis, the end sequela of chronic liver disease, is a leading cause of death in the United States and results in significant personal and societal burdens.1 Indeed, in the past decade, the prevalence of cirrhosis in the United States has doubled while annual mortality due to cirrhosis has increased by 65%.1 Cirrhosis is characterized by a myriad of debilitating and often fatal complications, including numerous hematologic derangements in all phases of hemostasis.2 Namely, cirrhosis results in a fall in both hepatically produced clotting factors and hepatically produced anticoagulants, dysfibrinogenemia, elevations in von Willebrand factor, factor VIII (FVIII), elevated tPA, and derangements in platelet function and count.3 Thrombocytopenia in cirrhosis itself is multifactorial and secondary to reduced thrombopoietin, splenic platelet sequestration, and hypersplenism.2 This array of derangements results in a “rebalanced” hemostasis in which pro- and anticoagulant dyscrasias offset one another.4 As a multifactorial phenomenon, however, this rebalance is not adequately reflected by routine laboratory tests commonly used to characterize cirrhotic coagulopathy, including PT/INR, which only assesses isolated reductions in procoagulant factors.3 Importantly, cirrhotic hemostasis is tenuous and easily derailed by common insults including infection, renal failure, endothelial dysfunction, and hemodynamic stressors such as portal hypertension.2,4 Hemodynamically significant bleeding and thrombosis are common in advanced liver disease (Figure 1).5
FIGURE 1.
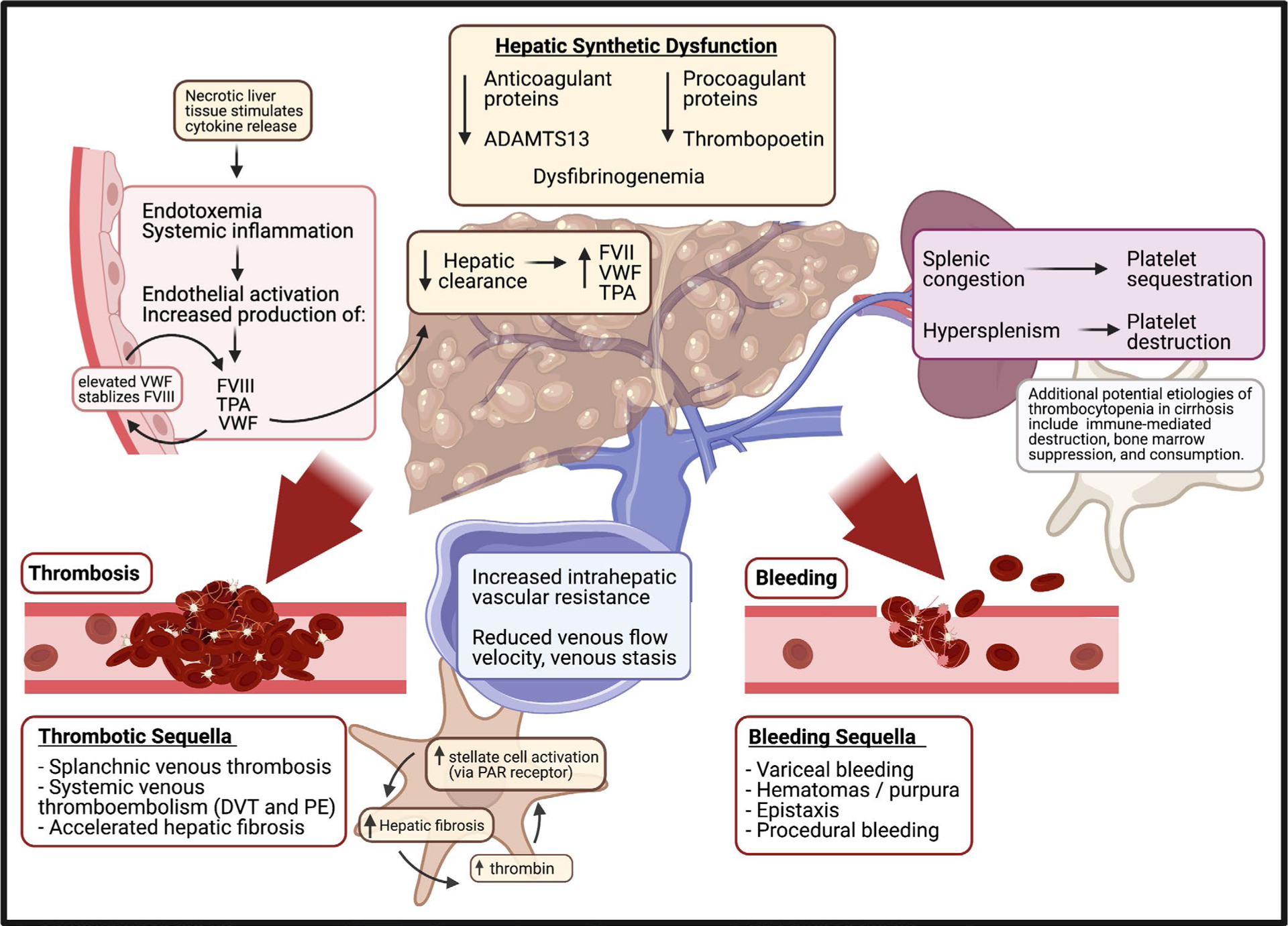
Pathogenesis of hematologic derangements of cirrhosis Created with biorender.com
Given the complexity and fragility of the rebalanced hemostasis of cirrhosis, uncertainty persists regarding best practices for the evaluation and management of hemostasis. Most fundamentally, concerns have been raised regarding the utility of routine hematologic tests including PT/INR, platelet count, PTT, and fibrinogen as both clinical markers and therapeutic targets.2,3,6 Growing evidence supports the use of functional evaluations of hemostasis in cirrhosis including thromboelastography.7 Commonly utilized transfusion thresholds are under increasing scrutiny, and recommendations regarding the use of procoagulant therapies in patients with liver disease continue to evolve.2,6 Additionally, a clear consensus on prevention and management of thrombotic complications of cirrhosis has yet to be reached.8,9
In summary, multiple unanswered questions remain regarding the optimal management of the hematologic sequalae of chronic liver disease. This review will summarize ongoing areas of investigation including hematologic markers, best transfusion practices, and pro- and anticoagulant therapies for patients with cirrhosis. We will also identify areas for potential continued research.
2 |. HEMOSTATIC DERANGEMENTS
2.1 |. Primary hemostasis—Platelets
As many as 77% of patients with cirrhosis have thrombocytopenia, although the thrombocytopenia is rarely severe and most patients maintain platelet counts >30 000–40 000.2,10 The pathogenesis of cirrhotic thrombocytopenia is a multifactorial imbalance between platelet production and destruction. Reduced thrombocytogenesis is primarily driven by low thrombopoietin (TPO), a key hormone regulator of megakaryocyte growth and platelet production that is normally secreted by hepatocytes at a constant rate.11 Patients with cirrhosis have been shown to have low hepatic TPO-mRNA and recovered TPO levels are observed following orthotopic liver transplantation, evidencing deficient hepatic production in cirrhosis.12 Insufficient platelet production is often also secondary to bone marrow suppression concomitant with the underlying etiology or sequela of liver disease including alcoholic bone marrow suppression, folate and B12 deficiencies, hepatitis C infection, and interferon treatment.2,12 Increased destruction in platelets is also a driver of thrombocytopenia. Not only does portal hypertension reduce circulating platelets via splenic sequestration, but also radiolabeled platelet studies in cirrhosis demonstrate reduced platelet survival times, suggesting increased destruction, likely driven by the hypersplenism seen in up to 55% of advanced liver disease patients.2,13 Immune-mediated platelet destruction is a known complication of hepatitis C or primary biliary cirrhosis. And more broadly, autoantibodies against GPIIb-IIIa and other platelet glycoproteins have been described in patients with cirrhosis, implicating auto-antibody mediated platelet destruction as another potential, but likely uncommon, cause of thrombocytopenia.14,15 Lastly, accelerated platelet consumption secondary to cirrhosis-related hypercoagulability, or chronic low-grade DIC, may also contribute.12,16 Despite its ubiquity, the thrombocytopenia of advanced liver disease is rarely predictive of increased bleeding risk.16,17
The functionality of platelets in cirrhosis is an area of ongoing research.12 Platelet function in terms of adhesion, activation, and aggregation—as measured via conventional metrics such as light aggregometry, PFA-100, and bleeding time—has traditionally been viewed as impaired.18–20 Notably, many platelet function assays require a platelet count >100 000 to be accurate. And more recently, evaluation using flow cytometry and other techniques has demonstrated preservation of all three functions when platelet count and hematocrit are adjusted to normal levels, suggesting that cirrhotic thrombocytopathy may not be significant in vivo.21,22 Platelets also function to provide a surface for the assembly of enzymatic complexes involved in thrombin formation.23 In this regard, assessments of platelet-supported thrombin generation in patients with cirrhosis demonstrate adequate, or even increased, thrombin production.4,23,24 Ultimately, similar to platelet count, the utility of platelet function tests in predicting bleeding risk in advanced liver disease is minimal, as platelet function may be physiologically preserved despite abnormal metrics.12,25
2.2 |. Primary hemostasis/Von Willebrand factor
Von Willebrand factor (VWF), a circulating endothelial-cell-produced multimeric protein that serves to adhere platelets to the site of vessel injury, is elevated in patients with cirrhosis. This may be due to endothelial damage, reduced liver-mediated clearance, or even direct synthesis in the cirrhotic liver.22,26 In any case, elevated VWF levels are a possible compensatory mechanism to the functional detriment of thrombocytopenia in cirrhosis and are strongly correlated to disease severity.3,12 Although the functional capacity of VWF declines as liver disease advances, the quantitative increase in VWF appears to overcome the qualitative defects as VWF-dependent platelet adhesion assays in cirrhosis demonstrate significantly elevated adhesion.22 Additionally, levels of ADAMTS13, a hepatically produced VWF cleaving protease, are commonly reduced in cirrhosis.27,28 Low ADAMTS13 levels reflect hepatic synthetic decline and are closely correlated with clinical outcome in advanced disease.29
2.3 |. Coagulation
With the exception of FVIII and VWF, pro- and anticoagulation factors are exclusively produced by liver parenchymal cells. Clotting factor levels fall in parallel with the progression of liver disease, as do the levels of proteins C and S, although they retain full functionality.4 In contrast, FVIII, one of the most potent drivers of thrombin generation, is produced in intra- and extra-hepatic endothelial cells and is often markedly increased in cirrhosis.3 This is due to increased synthesis—cytokine release from necrotic liver tissue stimulates endothelial production of FVIII—as well as reduced clearance.30 Namely, elevated VWF in cirrhosis binds to FVIII and prevents protease cleavage.30 Degradation is also impaired by reduced hepatic expression of low-density lipoprotein receptor, which mediates FVIII cellular uptake and eventual degradation.30
Reduced hepatic synthesis of coagulation factors in cirrhosis leads to well-known laboratory derangements in PT/INR. Although PT derangements reflect hepatocellular damage and have prognostic significance, these tests do not comprehensively reflect the rebalanced hemostasis of chronic liver disease. INR only reflects variations in procoagulant factors, but does not factor in a concomitant fall in anticoagulant factors or endothelial-based thrombosis modulators such as thrombomodulin.31 Conventional assessments of coagulation, therefore, do not reliably predict bleeding (including gastrointestinal and procedural bleeding), or thrombotic risk.30,32,33
In fact, thrombin generation tests done in the presence of thrombomodulin to reflect conditions in vivo demonstrate normal to increased thrombin generation in patients with cirrhosis.2,34 Partial resistance to thrombomodulin, which accelerates the thrombin-mediated conversion of protein C into its active form, has been demonstrated in cirrhosis and may contribute to preserved thrombin production.35 Reflecting this, thromboelastography (TEG) which measures the hemostatic characteristics of whole blood and takes into account clotting factor, platelet, and fibrin activity demonstrates normal hemostasis in patients with advanced liver disease.2,7,12,36 Although it should be acknowledged that TEG or Rho-TEM may be less suited to evaluate baseline hemostasis, and rather holds greater utility as a point of care test in instances of acute blood loss, as this is the primary situation in which most TEG results will be “abnormal.”
2.4 |. Fibrinolysis
Most pro- and antifibrinolytic proteins including plasminogen, a2antiplasmin, thrombin-activatable fibrinolysis inhibitor, and FXIII are synthesized in the liver and are reduced in chronic liver disease. Tissue plasminogen activator (tPA) and PAI-1 are the exceptions, as both are synthesized in endothelial cells, and in the case of PAI-1, multiple other tissues including adipose tissue.2 Similar to VWF, tPA levels in chronic liver disease are typically elevated due to enhanced endothelial production and reduced hepatic clearance. PAI-1 levels are variable; however, evidence of increased tPA activity in cirrhosis indicates that PAI-1 is decreased at least relative to TPA.37 There is some evidence that the net effect of these changes is to produce hyperfibrinolysis in cirrhosis, particularly given the potential effects of fibrinolytic ascitic fluid,38,39 although the reduction in antifibrinolytic proteins does appear to be largely balanced by a joint decrease in pro-fibrinolytic factors.3,40 Of note, antifibrinolytic therapy is commonly utilized during liver transplant surgery to prevent fibrinolysis and bleeding.41–43
2.5 |. Thrombosis
The rebalanced hemostasis of advanced liver disease is tenuous and is increasingly seen as more pro- than anti-thrombotic.35 Hematologic derangements including elevated VWF, resistance to thrombomodulin, reduced protein C, significantly elevated FVIII, and enhanced platelet-mediated thrombin production all contribute to a procoagulant state.35 Systemic processes including increased intra-hepatic vascular resistance, reduced venous flow velocity, venous stasis, and endothelial activation due to endotoxemia and systemic inflammation also precipitate coagulation.31,44 As such, cirrhosis is associated with an increased risk of splanchnic venous thrombosis and peripheral venous thromboembolism.30,45 Most estimates identify the prevalence of portal vein thrombosis in cirrhosis to be between 0.6% and 15%,46,47 although some studies show prevalence as high as 26% in end-stage liver disease.31,48 Post-transplant, portal vein thrombosis is linked to a 30% mortality increase.35 Venous thromboembolism (VTE), specifically deep vein thrombosis and/or pulmonary embolism, occurs in up to 6% of patients with cirrhosis, which is approximately doubled the risk of those without cirrhosis.49,50 VTE has been associated with increased mortality in compensated and decompensated patients with cirrhosis.51 As with splanchnic thrombosis, risk increases with disease severity and progressive hepatic synthetic dysfunction.49 Ultimately, however, the complete pathogenesis of thrombosis in cirrhosis remains undetermined, and investigations are confounded by an increase in other risk factors inherent to severe illness such as immobility and systemic inflammation.31
Evidence suggests that the procoagulant imbalance of chronic liver disease also contributes to hepatic fibrosis.3 For instance, in the setting of hepatitis C, patients with factor V Leiden mutation experience significantly accelerated fibrosis while those with hemophilia experience less severe fibrosis.35,52,53 Additionally, hepatic vein thrombi (Budd-Chiari syndrome) is recognized as a direct cause of hepatic fibrosis.54 The pathogenesis of hepatic fibrosis in the setting of hypercoagulability may be due to microthrombi leading to hypoxia and cellular injury.55 More likely, however, fibrosis is driven via direct stellate cell activation through thrombin binding to PAR-1 receptors and also possibly via factor Xa binding to PAR-2 receptors, both of which are overly expressed on cirrhotic stellate cells and are correlated with disease progression.35,52 Upon activation, quiescent stellate cells are converted to wound-healing myofibroblasts with robust pro-fibrotic activity.35 Highlighting the potentially contributory role of PAR receptors, PAR-1 is the primary receptor for thrombin in platelets and anti-platelet therapy has been associated with reduced fibrosis.52 Similarly, enoxaparin has been shown to slow hepatic decompensation (defined as ascites, sepsis, variceal bleeding, or encephalopathy) and improve survival in patients with portal vein thrombosis, likely via a reduction in thrombin and factor Xa PAR binding.56 The efficacy of direct-oral anticoagulation (DOAC) therapy or direct factor Xa inhibition in slowing fibrosis via a secondary reduction in downstream PAR activation is a potentially interesting area of future research.52
Beyond potential anti-fibrotic benefits, there is some evidence supporting the safety and efficacy of therapeutic anticoagulation (AC) in selected patients with cirrhosis (Table 1), particularly in patients with compensated cirrhosis, or Child-Pugh class A or B cirrhosis. Although the evidence is still developing, some retrospective and small prospective trials suggest that in patients with portal vein thrombosis (PVT), therapeutic AC does not increase bleeding risk (including variceal bleeding risk), leads to increased recanalization, and achieves reduced progression of thrombosis.45,57,58 In particular, PVT treatment and recanalization in patients preparing for liver transplant may be considered as significant increases in morality are associated with PVT in this setting.45,57,59 Low molecular weight heparin is safe and effective, as well as DOACs, which have been shown to be superior to warfarin for treatment of PVT in cirrhosis, although clinicians should remain mindful of the evidence’s relative limitations.57,60,61 Similarly, a 2018 systematic review demonstrated that therapeutic AC in patients with cirrhosis and atrial fibrillation achieves reduced stroke risk without an increased risk of bleeding.62 Although most research on management of venous thrombosis in cirrhosis has focused on PVT, best practice recommendations extrapolate from the success of PVT treatment and recommend systemic heparin infusion for symptomatic peripheral VTE in patients with cirrhosis in the acute setting,.45
TABLE 1.
Thrombosis prophylaxis and treatment in cirrhosis
| Prophylaxis | ||
|---|---|---|
| Patient population | Agent | Comments |
| Hospitalized patients | LMWH | The best practice guidelines recommend prophylactic anticoagulation in hospitalized patients with cirrhosis, although ultimately, the decision is still often left up to clinician gestalt.45,59,75 |
| Patients awaiting liver transplantation | PVT prophylaxis is indicated among patients awaiting transplantation.3,59,74 | |
| General cirrhotic population. | Long-term prophylaxis has been shown to successfully prevent PVT and may decrease decompensation without increasing bleeding, although prospective trials are lacking. Decisions should be made on a case-by-case basis.45,56 *No published data on DOACs in this space to date. |
|
| Treatment | ||
| Thrombosis | Agent | Comment |
| Portal vein thrombosis (PVT) | Therapeutic anticoagulation is indicated in liver transplantation candidates and patients with symptomatic PVTs. Other treatment decisions via a case-by-case basis, including for peripheral VTEs.45 The treatment should continue to at least 6 mo prior to repeat imaging.59 | |
| Splanchnic vein thrombosis | LMWH | Heparin infusion can be utilized initially with transition to LMWH if tolerated. The use of heparin products is challenged by low antithrombin III and variability in the—Xa assay. |
| Peripheral VTEs | DOAC | Indicated in compensated cirrhosis. Superior to warfarin—confer equal or reduced bleeding risk without monitoring requirements.59–61 Caution given component of hepatic metabolism. |
| Atrial fibrillation | DOAC | Anticoagulation has been shown to reduce stroke without increased risk of bleeding.62 |
No published data on DOACs in this space to date.
In terms of choice of agents, LMWH has traditionally been favored in cirrhosis, although variable levels of anti-Xa in liver disease (due to reduced levels of antithrombin and variable laboratory practices re exogenous ATIII supplementation) make interpretation of the anti-Xa assay typically used for evaluation challenging.63,64 Vitamin K antagonists are also frequently utilized, although they are hindered by a counterproductive reduction protein C and reliance on poorly representative INR, leading to significant time potentially spent outside of the therapeutic range. The aPTT assay, used to assess unfractionated heparin, is also skewed in cirrhosis. Although patients with liver disease were not included in the major clinical trials demonstrating DOAC safety and efficacy, DOACs may be preferred for AC in the setting of liver disease. Subsequent investigations of therapeutic AC with DOACs in cirrhosis have shown that they confer an equal or even reduced bleeding risk, particularly central nervous system bleeding risk, compared with traditional anticoagulants, without the need for stringent monitoring.8,65–68 Multiple randomized controlled trials in patients with PVT have demonstrated DOAC superiority over warfarin in terms of reducing clot burden and mortality.59–61 Notably, however, all DOACs undergo some form of hepatic metabolism, and while evidence suggests they do not carry an elevated risk of serious hepatic injury, further adequately powered studies are warranted.69,70
There is no clear consensus on primary prevention of thromboses in cirrhosis (Table 1). Multiple studies have resulted in neither decrease in VTE nor increase in bleeding in hospitalized patients with cirrhosis who were placed on prophylactic AC.9,71,72 Long-term prophylaxis with enoxaparin, however, has been shown to successfully prevent PVT and decrease cirrhotic decompensation without increasing bleeding.56 Indeed, prevention of PVT may even achieve long-term reductions in bleeding risk through lowering portal venous pressure.73 In particular, given the adverse effects of PVT on liver transplantation, PVT prophylaxis is indicated among patients awaiting transplantation.3,59,74 The best practice guidelines also recommend prophylactic anticoagulation for peripheral DVT in hospitalized patients with cirrhosis, although ultimately the decision is still often left up to clinician gestalt.59,75
2.6 |. Bleeding
Although thrombotic risk may be more severe, multiple hematologic derangements of cirrhosis including reduced clotting factors, thrombocytopenia, and hyperfibrinolysis can contribute to a bleeding diathesis and bleeding complications are common. Variceal bleeding is the most prevalent and significant complication and occurs in up to 35% of patients with advanced liver disease.4,76 Other common presentations include bruising, purpura, epistaxis, menorrhagia, and procedural bleeding. Notably, many non-surgical invasive procedures (including liver biopsy, paracentesis, thoracentesis, transesophageal echocardiography (TEE), central vein catheter insertion, endoscopic esophageal varices ligation, and transarterial chemoembolization) are safe and not associated with increased bleeding risk in cirrhosiss.77 This is reflective of the overall “rebalance” of hemostasis. Indeed, in contrast to thrombosis, most bleeding in cirrhosis is thought to be secondary to systemic, hemodynamic, and vascular abnormalities including endotoxemia, endothelial dysfunction, renal failure, disseminated intravascular coagulation, and portal hypertension rather than coagulopathies.4,76–79 This is true of variceal bleeding and may also be the case for other common bleeding sequalae, including those related to most invasive procedures.4,76,77,80
The prevention and treatment of bleeding in cirrhosis is nuanced. Blood product transfusions deliver substantial volumes of oncotically active fluid and can exacerbate portal hypertension through circulatory overload as well as increase risk of infection and transfusion reactions6,59 Common tests to evaluate coagulation including PT/INR, PTT, platelet count, and fibrinogen level are often inaccurate predictors of bleeding risk as they do not reflect more clinically relevant hemodynamic risk factors.81 For this reason, the utility of routine evaluation and correction of coagulation indices to addressing bleeding risk is increasingly questioned and guidelines recommend sparing use of blood products in cirrhosis.59,77,79 However, preventive or therapeutic correction of some coagulopathies is beneficial in certain settings (Table 2).
TABLE 2.
Bleeding prophylaxis and treatment in cirrhosis
| Prophylaxis | ||||
|---|---|---|---|---|
| High risk | Target | Value | Agent(s) | Comment |
| High-risk procedures: Major surgery* Intra-cranial or spinal procedures |
Platelet count | >50 000* | Platelet transfusion Oral thrombopoietin agonists |
TPO is superior to platelet transfusion as it does not increase portal pressure and results in more prolonged elevations in platelet count. However, TPO requires 10 d to take effect and does carry increased risk of PVT, although newer agents including avatrombopag and lusutrombopag seem to mitigate this risk.45,59
*liver transplant surgery is the exception and can be safety carried out with platelet counts <50 00045 |
| Fibrinogen | >120 mg/dL | Cryoprecipitate Fresh-frozen plasma |
No controlled trials exist, but in the acute high-risk presurgical setting, correction is warranted to create additional substrate for clot formation. For this purpose, cryoprecipitate is preferred over FFP as it is delivered in smaller volume and less likely to exacerbate portal HTN.6,45,59 | |
| Tranexamic acid (TXA) | Prophylactic use prior to liver transplantation has demonstrated successful reductions in blood loss and transfusion requirements, although not in mortality.41–43 | |||
| INR | N/A | Correction not recommended. | There is no specific INR cutoff above which bleeding can be reliably predicted.102 | |
| FFP | Not recommended for use. Requires high transfusion volume, significant effects on portal pressure limit use.59 | |||
| Prothrombin complex | The use is limited due to monitoring and dosage dependency on INR, although low volume of administration minimizes risk of the use. The literature on the use in cirrhosis is limited.59 | |||
| Vitamin K | Requires >12 h to exert an effect. Paucity of data available to guide patient selection, and dosing. May be useful in patients with malnutrition or prolonged antibiotic therapy.59 | |||
| TEG | N/A | Although holistic evaluations of coagulation can meaningfully guide transfusion strategies on a case-by-case basis, validated target levels do not yet exist.59 | ||
| Low risk | Target | Value | Agent(s) | Comment |
| Paracentesis Thoracentesis |
Platelet count | N/A | Routine evaluation and correction are not recommended in this setting. | |
| Routine endoscopy | INR | N/A | ||
| Treatment | Agent | Comment | ||
| PRBC transfusion | Transfusion threshold of Hb 7 improves outcomes in cirrhotic patients with upper GI bleed.82 | |||
| Recombinant factor VIIa | Randomized controlled trials have not demonstrated benefit in terms of cessation or reduction of bleeding, or short-term mortality, although may have long-term benefit in the treatment of massive variceal hemmorhage.6,103,104 | |||
| Desmopressin | Enhances platelet function in uremia. Recommended for bleeding in patients with concomitant renal failure. Lacks evidence for use in isolated cirrhosis, in which it has not been demonstrated to reduce bleeding.2,45,59 | |||
| Tranexamic acid | Not believed to generate a hypercoagulable state, although may exacerbate existing thrombi. Typically utilized for postprocedural bleeding.45,59 | |||
| Aminocaproic acid | ||||
Liver transplant surgery is the exception and can be safely carried out with platelet counts <50,000.
In terms of hemoglobin, packed red blood cell (PRBC) transfusion prior to high-risk procedures or major surgery is recommended to maintain hematocrit >25% in cirrhosis, as this is thought to improve platelet margination.59 If PRBC transfusion is required in the setting of active bleeding, a restrictive transfusion threshold of hemoglobin of 7 is known to significantly improve outcomes of upper GI bleeding, particularly in patients with cirrhosis.82 In terms of platelets, in the event of an upcoming high-risk procedure or surgery, targeting a platelet count of 50 000–60 000 either via TPO administration or platelet transfusion is suggested.45,59 Platelet-supported thrombin generation has been shown to be preserved at platelet counts >56 000.23 However, no clinical trials have demonstrated improved hemostasis with prophylactic platelet transfusions to a threshold of 50 000. Importantly, thoracentesis, paracentesis, or upper endoscopy for variceal banding have been shown to be safe at platelet counts as low as 20 000 and for which prophylactic platelet transfusion is not recommended.59,83 For the correction of thrombocytopenia, TPO may be preferred over platelet transfusion when possible, although it requires approximately 10 days to achieve full effect.59 TPO does not exacerbate portal hypertension, provides longer-term platelet supplementation, and is significantly more cost-effective than platelet transfusions (one course of avatrombopag costs approximately $3800, vs. one unit platelet transfusion costs approximately $9200).84,85 Beyond time constraints, the major hindrance of TPO in cirrhosis is a potential increased risk of PV, although newer agents including avatrombopag and lusutrombopag may have lesser or no risk.59,86–89 additionally, much is yet to be known regarding optimal dosing to minimize the adverse thrombotic effects.2,86,87 The maintenance of fibrinogen levels above 100–120 mg/dL has also demonstrated reduction in bleeding and mortality in cirrhosis.6,90 Cryoprecipitate is superior to fresh frozen plasma for this purpose as it does not require large transfusion volumes.6 Lastly, correction of hyperfibrinolysis is potentially beneficial; prophylactic use of transexamic acid (TXA) prior to liver transplantation has demonstrated successful reductions in blood loss and transfusion requirements, although not in mortality.41–43 Notably, TXA has not been found to be effective for the management of acute variceal bleeding, although it may be useful for the management of diffuse mucosal bleeding.45,91 Further investigation regarding adverse events and optimal dosing of TXA in cirrhosis is warranted.6
In contrast to hemoglobin, platelets, and fibrinogen, INR-based transfusion targets are not well supported.59,92 INR is not predictive of bleeding risk in cirrhosis, and its correction to an arbitrary target typically requires large volumes of FFP, which exacerbate portal hypertension while achieving minimal, or even reduced, thrombin generation.59,92,93 Additionally, FFP is time consuming to administer and may expose patients to unnecessary procedural delay.2 Prothrombin complex concentrate (PCC) has also been shown to effectively reduce INR in patients with cirrhosis and can be administered significantly more quickly and in smaller volume than FFP.6 However, evidence is scarce, and the degree of reduction in bleeding is unclear, while the risk of thromboembolism and DIC is not insignificant.6,94 The clinical utility of PCC in liver disease is likely in emergency bleeding, although much remains unknown. The use of recombinant factor VIIa infusion to correct INR is also not recommended as it has not demonstrated reduction in intra-operative transfusion requirements or mortality.6,43,95 Vitamin K is also often administered to address PT/INR elevations in advanced liver disease patients; however, there are little data to support its use.2,96,97 DDAVP has been considered as another potential hemostatic agent in patients with cirrhosis; however, there is no evidence that it improves outcomes in variceal bleeding or reduces intra-operative transfusion requirements.2,59,98 Some guidelines do recommend the use of DDAVP for the treatment of active bleeding in patients with concomitant uremic renal failure.59
Beyond targeting individual coagulation components, the use of TEG or other viscoelastic tests of whole blood coagulation to guide peri- and intra-operative transfusion during liver transplant has been shown to successfully reduce bleeding, but without definitive mortality benefit.6,36 Although most studies are small and limited, and largely included procedures with low-to-moderate bleeding risk, TEG has also been successfully used to predict post-transplant thrombosis risk, bleeding risk during invasive procedures, and to reduce the use of blood components in non-variceal upper gastrointestinal bleeds without increasing bleeding complications.36,99–101 Ultimately, the clinical use of TEG in liver disease is still limited by subjective interpretation due to lack of validated target levels.59
3 |. CONCLUSION
Cirrhosis is a state of tenuously rebalanced both pro- and anti-thrombotic hematologic derangements. This rebalanced hemostasis is likely more pro- than anti-thrombotic, and in addition, to driving clot formation may also directly contribute to hepatic fibrosis. Although definitive evidence is lacking, AC appears to be indicated for select patient populations with cirrhosis, including patients with symptomatic PVT, patients awaiting liver transplant, and some patients with peripheral thromboses. However, most studies demonstrating benefit with AC are small and retrospective, with heavily selected patient populations. No prospective randomized controlled studies exist, leaving a lack of clear guidance for commonplace clinical questions. In particular, given the high prevalence and significant heterogeneity of splanchnic venous thrombosis, clarifying indications for AC in this setting would be especially useful. Additionally, most data on AC in patients with cirrhosis are based on LMWH which, given monitoring concerns (anti-Xa assays), expense, and cumbersome administration, has limited long-term utility. There may be a role for DOAC’s to expand the favorable data from LMWH, further exploration in this area has the potential to significantly impact daily practice.
In terms of bleeding sequela, one particularly interesting area of continued investigation may be in further clarifying and expanding the use of TPO memetics. Evidence suggests that preprocedural TPO is associated with fewer platelet transfusions and less bleeding.89 However, current data are limited by significant lack of standardization in transfusion practices, paucity of data prior to high-risk surgeries or procedures, and concern regarding potential thrombosis-related adverse events.89 Notably, minimization of transfusion burden is also beneficial in that it mitigates sensitization prior to transplant, which complicates cross-matching for future transfusions. Another avenue of highly clinically relevant investigation is the evaluation of global hemostasis in cirrhosis. Although currently available measurements of whole blood functionality are useful, everyday clinical use is limited by the lack of validated, easy to interpret, and standardized targets. The clarification of such targets would be foundational to the standardization and protocolization of the management the coagulopathy of chronic liver disease, a much-needed development given the current wide diversity of practice. In sum, although much recent progress has been made in advancing the understanding of the hematologic situation in cirrhosis, major questions remain. The development of evidenced-based guidelines for the management of common clinical scenarios, both in terms of bleeding and thrombosis should remain a priority.
Novelty Statement:
This paper presents an up-to-date review of the available literature regarding the hemostatic and thrombotic sequela of chronic liver disease.
The liver disease leads to numerous derangements in blood proteins and consequently carries an increased risk of both bleeding and clotting.
This review provides guidance regarding the pathophysiology, monitoring, prevention, and management of coagulation disorders and related complications in liver disease.
SIGNIFICANCE STATEMENT.
This paper provides a review of the pathophysiology and management of the blood disorders associated with chronic liver disease. Liver disease leads to numerous derangements in blood proteins and consequently carries an increased risk of both bleeding and clotting. Liver disease also affects the accuracy of common tests of blood function. This review succinctly summarizes available literature to provide guidance regarding monitoring, prevention, and management of blood disorders and related complications in liver disease.
Footnotes
CONFLICT OF INTEREST
None.
DATA AVAILABILITY STATEMENT
Data sharing not applicable – no new data generated Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1.Tapper EB, Parikh ND. Mortality due to cirrhosis and liver cancer in the United States, 1999–2016: observational study. BMJ. 2018;362:k2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kujovich JL. Coagulopathy in liver disease: a balancing act. Hematology. 2015;2015(1):243–249. [DOI] [PubMed] [Google Scholar]
- 3.Tripodi A, Mannucci PM. The Coagulopathy of chronic liver disease. N Engl J Med. 2011;365(2):147–156. [DOI] [PubMed] [Google Scholar]
- 4.Lisman T, Porte RJ. Rebalanced hemostasis in patients with liver disease: evidence and clinical consequences. Blood. 2010;116(6):878–885. [DOI] [PubMed] [Google Scholar]
- 5.Rodríguez-Castro KI, Antonello A, Ferrarese A. Spontaneous bleeding or thrombosis in cirrhosis: What should be feared the most? World J Hepatol. 2015;7(14):1818–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu P, Hum J, Jou J, Scanlan RM, Shatzel J. Transfusion strategies in patients with cirrhosis. Eur J Haematol. 2020;104(1):15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hum J, Amador D, Shatzel JJ, et al. Thromboelastography better reflects hemostatic abnormalities in cirrhotics compared with the international normalized ratio. J Clin Gastroenterol. 2020;54(8):741–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hum J, Jou J, Deloughery TG, Shatzel J. The effectiveness and safety of Direct Oral Anticoagulants (DOACs) Vs. Traditional anticoagulants in patients with cirrhosis. Blood. 2016;128(22):5015. [Google Scholar]
- 9.Shatzel J, Dulai PS, Harbin D, et al. Safety and efficacy of pharmacological thromboprophylaxis for hospitalized patients with cirrhosis: a single-center retrospective cohort study. J Thromb Haemost. 2015;13(7):1245–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qamar AA, Grace ND, Groszmann RJ, et al. Incidence, prevalence, and clinical significance of abnormal hematologic indices in compensated cirrhosis. Clin Gastroenterol Hepatol. 2009;7(6):689–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller JB, Figueroa EJ, Haug RM, Shah NL. Thrombocytopenia in chronic liver disease and the role of thrombopoietin agonists. Gastroenterol Hepatol (N Y). 2019;15(6):326–332. [PMC free article] [PubMed] [Google Scholar]
- 12.Violi F, Basili S, Raparelli V, Chowdary P, Gatt A, Burroughs AK. Patients with liver cirrhosis suffer from primary haemostatic defects? Fact or fiction? J Hepatol. 2011;55(6):1415–1427. [DOI] [PubMed] [Google Scholar]
- 13.Li L, Duan M, Chen W, et al. The spleen in liver cirrhosis: revisiting an old enemy with novel targets. J Transl Med. 2017;15(1):111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kajihara M, Kato S, Okazaki Y, et al. A role of autoantibody-mediated platelet destruction in thrombocytopenia in patients with cirrhosis. Hepatology. 2003;37(6):1267–1276. [DOI] [PubMed] [Google Scholar]
- 15.Pereira J, Accatino L, Alfaro J, Brahm J, Hidalgo P, Mezzano D. Platelet autoantibodies in patients with chronic liver disease. Am J Hematol. 1995;50(3):173–178. [DOI] [PubMed] [Google Scholar]
- 16.Senzolo M, Burra P, Cholongitas E, Burroughs AK. New insights into the coagulopathy of liver disease and liver transplantation. World J Gastroenterol. 2006;12(48):7725–7736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Afdhal N, McHutchison J, Brown R, et al. Thrombocytopenia associated with chronic liver disease. J Hepatol. 2008;48(6):1000–1007. [DOI] [PubMed] [Google Scholar]
- 18.Vinholt PJ, Hvas A-M, Nielsen C, et al. Reduced platelet activation and platelet aggregation in patients with alcoholic liver cirrhosis. Platelets. 2018;29(5):520–527. [DOI] [PubMed] [Google Scholar]
- 19.Lisman T, Leebeek FWG, de Groot PG. Haemostatic abnormalities in patients with liver disease. J Hepatol. 2002;37(2):0168–8278. [DOI] [PubMed] [Google Scholar]
- 20.Escolar G, Cases A, Vinas M, et al. Evaluation of acquired platelet dysfunctions in uremic and cirrhotic patients using the platelet function analyzer (PFA-100): influence of hematocrit elevation. Haematologica. 1999;84(7):614–619. [PubMed] [Google Scholar]
- 21.Lisman T, Adelmeijer J, De Groot PG, Janssen HLA, Leebeek FWG. No evidence for an intrinsic platelet defect in patients with liver cirrhosis – studies under flow conditions. J Thromb Haemost. 2006;4(9):2070–2072. [DOI] [PubMed] [Google Scholar]
- 22.Lisman T, Bongers TN, Adelmeijer J, et al. Elevated levels of von Willebrand Factor in cirrhosis support platelet adhesion despite reduced functional capacity. Hepatology. 2006;44(1):53–61. [DOI] [PubMed] [Google Scholar]
- 23.Tripodi A, Primignani M, Chantarangkul V, et al. Thrombin generation in patients with cirrhosis: The role of platelets. Hepatology. 2006;44(2):440–445. [DOI] [PubMed] [Google Scholar]
- 24.Gatt A, Riddell A, Calvaruso V, Tuddenham EG, Makris M, Burroughs AK. Enhanced thrombin generation in patients with cirrhosis-induced coagulopathy. J Thromb Haemost. 2010;8(9):1994–2000. [DOI] [PubMed] [Google Scholar]
- 25.Lisman T, Porte RJ. Platelet function in patients with cirrhosis. J Hepatol. 2012;56(4):993–994. [DOI] [PubMed] [Google Scholar]
- 26.Hollestelle MJ, Geertzen HG, Straatsburg IH, van Gulik TM, van Mourik JA. Factor VIII expression in liver disease. Thromb Haemost. 2004;91(2):267–275. [DOI] [PubMed] [Google Scholar]
- 27.Feys HB, Canciani MT, Peyvandi F, Deckmyn H, Vanhoorelbeke K, Mannucci PM. ADAMTS13 activity to antigen ratio in physiological and pathological conditions associated with an increased risk of thrombosis. Br J Haematol. 2007;138(4):534–540. [DOI] [PubMed] [Google Scholar]
- 28.Mannucci PM, Canciani MT, Forza I, Lussana F, Lattuada A, Rossi E. Changes in health and disease of the metalloprotease that cleaves von Willebrand factor. Blood. 2001;98(9):2730–2735. [DOI] [PubMed] [Google Scholar]
- 29.Takaya H, Uemura M, Fujimura Y, et al. ADAMTS13 activity may predict the cumulative survival of patients with liver cirrhosis in comparison with the Child-Turcotte-Pugh score and the Model for End-Stage Liver Disease score. Hepatol Res. 2012;42(5):459–472. [DOI] [PubMed] [Google Scholar]
- 30.Roberts LN, Patel RK, Arya R. Haemostasis and thrombosis in liver disease. Br J Haematol. 2010;148(4):507–521. [DOI] [PubMed] [Google Scholar]
- 31.Hugenholtz GCG, Northup PG, Porte RJ, Lisman T. Is there a rationale for treatment of chronic liver disease with antithrombotic therapy? Blood Rev. 2015;29(2):127–136. [DOI] [PubMed] [Google Scholar]
- 32.Flores B, Trivedi HD, Robson SC, Bonder A. Hemostasis, bleeding and thrombosis in liver disease. J Transl Sci. 2017;3(3). Epub ahead of print. 10.15761/JTS.1000182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Segal JB, Dzik WH. Paucity of studies to support that abnormal coagulation test results predict bleeding in the setting of invasive procedures: an evidence-based review. Transfusion. 2005;45(9):0041–1132. [DOI] [PubMed] [Google Scholar]
- 34.Tripodi A, Salerno F, Chantarangkul V, et al. Evidence of normal thrombin generation in cirrhosis despite abnormal conventional coagulation tests. Hepatology. 2005;41(3):553–558. [DOI] [PubMed] [Google Scholar]
- 35.Tripodi A, Anstee QM, Sogaard KK, Primignani M, Valla DC. Hypercoagulability in cirrhosis: causes and consequences. J Thromb Haemost. 2011;9(9):1713–1723. [DOI] [PubMed] [Google Scholar]
- 36.Harrison MF. The misunderstood coagulopathy of liver disease: A review for the acute setting. West J Emerg Med. 2018;19(5):863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zermatten MG, Fraga M, Moradpour D, et al. Hemostatic alterations in patients with cirrhosis: From primary hemostasis to fibrinolysis. Hepatology. 2020;71(6):2135–2148. [DOI] [PubMed] [Google Scholar]
- 38.Rijken DC, Kock EL, Guimarães AH, et al. Evidence for an enhanced fibrinolytic capacity in cirrhosis as measured with two different global fibrinolysis tests. J Thromb Haemost. 2012;10(10):1538–7836. [DOI] [PubMed] [Google Scholar]
- 39.Agarwal S, Joyner KA, Swaim MW. Ascites fluid as a possible origin for hyperfibrinolysis in advanced liver disease. Am J Gastroenterol. 2000;95(11):3218–3224. [DOI] [PubMed] [Google Scholar]
- 40.Lisman T, Leebeek FWG, Mosnier LO, et al. Thrombin-activatable fibrinolysis inhibitor deficiency in cirrhosis is not associated with increased plasma fibrinolysis. Gastroenterology. 2001;121(1):131–139. [DOI] [PubMed] [Google Scholar]
- 41.Boylan JF, Klinck JR, Sandler AN, et al. Tranexamic acid reduces blood loss, transfusion requirements, and coagulation factor use in primary orthotopic liver transplantation. Anesthesiology. 1996;85(5):1043–1048. [DOI] [PubMed] [Google Scholar]
- 42.Dalmau A, Sabaté A, Acosta F, et al. Tranexamic acid reduces red cell transfusion better than epsilon-aminocaproic acid or placebo in liver transplantation. Anesth Analg. 2000;91(1):29–34. [DOI] [PubMed] [Google Scholar]
- 43.Gurusamy KS, Davidson BR. Methods to decrease blood loss and transfusion requirements for liver transplantation. Cochrane Database Syst Rev. 2011;3. 10.1002/14651858.CD009052.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mantaka A, Augoustaki A, Kouroumalis EA, Samonakis DN. Portal vein thrombosis in cirrhosis: diagnosis, natural history, and therapeutic challenges. Ann Gastroenterol. 2018;31(3):315–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Intagliata NM, Argo CK, Stine JG, Lisman T, Caldwell SH, Violi F. Concepts and controversies in haemostasis and thrombosis associated with liver disease: Proceedings of the 7th international coagulation in liver disease conference. Thromb Haemost. 2018;118(08):1491–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amitrano L, Anna Guardascione M, Brancaccio V, et al. Risk factors and clinical presentation of portal vein thrombosis in patients with liver cirrhosis. J Hepatol. 2004;40(5):736–741. [DOI] [PubMed] [Google Scholar]
- 47.Northup PG, McMahon MM, Ruhl AP, et al. Coagulopathy does not fully protect hospitalized cirrhosis patients from peripheral venous thromboembolism. Am J Gastroenterol. 2006;101(7):1524–1528. [DOI] [PubMed] [Google Scholar]
- 48.Tripodi A, Primignani M, Chantarangkul V, et al. An imbalance of pro- vs anti-coagulation factors in plasma from patients with cirrhosis. Gastroenterology. 2009;137(6):2105–2111. [DOI] [PubMed] [Google Scholar]
- 49.Dabbagh O, Oza A, Prakash S, Sunna R, Saettele TM. Coagulopathy does not protect against venous thromboembolism in hospitalized patients with chronic liver disease. Chest. 2010;137(5):1145–1149. [DOI] [PubMed] [Google Scholar]
- 50.Sogaard KK, Horvath-Puho E, Gronbaek H, Jepsen P, Vilstrup H, Sorensen HT. Risk of venous thromboembolism in patients with liver disease: a nationwide population-based case-control study. Am J Gastroenterol. 2009;104(1):96–101. [DOI] [PubMed] [Google Scholar]
- 51.Wu H, Nguyen GC. Liver cirrhosis is associated with venous thromboembolism among hospitalized patients in a nationwide US study. Clin Gastroenterol Hepatol. 2010;8(9):800–805. [DOI] [PubMed] [Google Scholar]
- 52.Pant A, Kopec AK, Luyendyk JP. Role of the blood coagulation cascade in hepatic fibrosis. Am J Physiol Gastrointest Liver Physiol. 2018;315(2):G171–G176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wright M, Goldin R, Hellier S, et al. Factor V Leiden polymorphism and the rate of fibrosis development in chronic hepatitis C virus infection. Gut. 2003;52(8):1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tanaka M, Wanless IR. Pathology of the liver in budd-chiari syndrome: Portal vein thrombosis and the histogenesis of veno-centric cirrhosis, venoportal cirrhosis, and large regenerative nodules. Hepatology. 1998;27(2):488–496. [DOI] [PubMed] [Google Scholar]
- 55.Anstee QM, Wright M, Goldin R, Thursz MR. Parenchymal extinction: coagulation and hepatic fibrogenesis. Clin Liver Dis. 2009;13(1):117–126. [DOI] [PubMed] [Google Scholar]
- 56.Villa E, Camma C, Marietta M, et al. Enoxaparin prevents portal vein thrombosis and liver decompensation in patients with advanced cirrhosis. Gastroenterology. 2012;143(5):1253–1260 e1254. [DOI] [PubMed] [Google Scholar]
- 57.Loffredo L, Pastori D, Farcomeni A, Violi F. Effects of anticoagulants in patients with cirrhosis and portal vein thrombosis: A systematic review and meta-analysis. Gastroenterology. 2017;153(2):480–487 e481. [DOI] [PubMed] [Google Scholar]
- 58.Delgado MG, Seijo S, Yepes I, et al. Efficacy and safety of anticoagulation on patients with cirrhosis and portal vein thrombosis. Clin Gastroenterol Hepatol. 2012;10(7):776–783. [DOI] [PubMed] [Google Scholar]
- 59.O’Leary JG, Greenberg CS, Patton HM, Caldwell SH. AGA clinical practice update: Coagulation in cirrhosis. Gastroenterology. 2019;157(1):34–43 e31. [DOI] [PubMed] [Google Scholar]
- 60.Hanafy AS, Abd-Elsalam S, Dawoud MM. Randomized controlled trial of rivaroxaban versus warfarin in the management of acute non-neoplastic portal vein thrombosis. Vascul Pharmacol. 2019;113:86–91. [DOI] [PubMed] [Google Scholar]
- 61.Nagaoki Y, Aikata H, Daijyo K, et al. Efficacy and safety of edoxaban for treatment of portal vein thrombosis following danaparoid sodium in patients with liver cirrhosis. Hepatol Res. 2018;48(1):51–58. [DOI] [PubMed] [Google Scholar]
- 62.Chokesuwattanaskul R, Thongprayoon C, Bathini T, et al. Efficacy and safety of anticoagulation for atrial fibrillation in patients with cirrhosis: A systematic review and meta-analysis. Dig Liver Dis. 2019;51(4):489–495. [DOI] [PubMed] [Google Scholar]
- 63.Khoury T, Ayman AR, Cohen J, Daher S, Shmuel C, Mizrahi M. The complex role of anticoagulation in cirrhosis: An updated review of where we are and where we are going. Digestion. 2016;93(2):149–159. [DOI] [PubMed] [Google Scholar]
- 64.Bechmann LP, Sichau M, Wichert M, Gerken G, Kroger K, Hilgard P. Low-molecular-weight heparin in patients with advanced cirrhosis. Liver Int. 2011;31(1):75–82. [DOI] [PubMed] [Google Scholar]
- 65.Pastori D, Lip GYH, Farcomeni A, et al. Incidence of bleeding in patients with atrial fibrillation and advanced liver fibrosis on treatment with vitamin K or non-vitamin K antagonist oral anticoagulants. Int J Cardiol. 2018;264:58–63. [DOI] [PubMed] [Google Scholar]
- 66.Steuber TD, Howard ML, Nisly SA. Direct oral anticoagulants in chronic liver disease. Ann Pharmacother. 2019;53(10):1042–1049. [DOI] [PubMed] [Google Scholar]
- 67.Intagliata NM, Henry ZH, Maitland H, et al. Direct oral anticoagulants in cirrhosis patients pose similar risks of bleeding when compared to traditional anticoagulation. Dig Dis Sci. 2016;61(6):1721–1727. [DOI] [PubMed] [Google Scholar]
- 68.Hum J, Shatzel JJ, Jou JH, Deloughery TG. The efficacy and safety of direct oral anticoagulants vs traditional anticoagulants in cirrhosis. Eur J Haematol. 2017;98(4):393–397. [DOI] [PubMed] [Google Scholar]
- 69.Qamar A, Vaduganathan M, Greenberger NJ, Giugliano RP. Oral anticoagulation in patients with liver disease. J Am Coll Cardiol. 2018;71(19):2162–2175. [DOI] [PubMed] [Google Scholar]
- 70.Douros A, Azoulay L, Yin H, Suissa S, Renoux C. Non-Vitamin K antagonist oral anticoagulants and risk of serious liver injury. J Am Coll Cardiol. 2018;71(10):1105–1113. [DOI] [PubMed] [Google Scholar]
- 71.Gómez Cuervo C, Bisbal Pardo O, Pérez-Jacoiste Asín MA. Efficacy and safety of the use of heparin as thromboprophylaxis in patients with liver cirrhosis: A systematic review and meta-analysis. Thromb Res. 2013;132(4):414–419. [DOI] [PubMed] [Google Scholar]
- 72.Yerke J, Bauer SR, Bass S, et al. Effectiveness of venous thromboembolism prophylaxis in patients with liver disease. World J Hepatol. 2019;11(4):379–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ageno W, Dentali F, Squizzato A. How I treat splanchnic vein thrombosis. Blood. 2014;124(25):3685–3691. [DOI] [PubMed] [Google Scholar]
- 74.Englesbe MJ, Kubus J, Muhammad W, et al. Portal vein thrombosis and survival in patients with cirrhosis. Liver Transpl. 2010;16(1):83–90. [DOI] [PubMed] [Google Scholar]
- 75.Intagliata NM, Henry ZH, Shah N, Lisman T, Caldwell SH, Northup PG. Prophylactic anticoagulation for venous thromboembolism in hospitalized cirrhosis patients is not associated with high rates of gastrointestinal bleeding. Liver Int. 2014;34(1):26–32. [DOI] [PubMed] [Google Scholar]
- 76.Sharara AI, Rockey DC. Gastroesophageal variceal hemorrhage. N Engl J Med. 2001;345(9):669–681. [DOI] [PubMed] [Google Scholar]
- 77.Schepis F, Turco L, Bianchini M, Villa E. Prevention and management of bleeding risk related to invasive procedures in cirrhosis. Semin Liver Dis. 2018;38(3):215–229. [DOI] [PubMed] [Google Scholar]
- 78.Basili S, Raparelli V, Violi F. The coagulopathy of chronic liver disease: Is there a causal relationship with bleeding? Yes. Eur J Intern Med. 2010;21(2):62–64. [DOI] [PubMed] [Google Scholar]
- 79.Tripodi A The coagulopathy of chronic liver disease: Is there a causal relationship with bleeding? No. Eur J Intern Med. 2010;21(2):65–69. [DOI] [PubMed] [Google Scholar]
- 80.Boks AL, Brommer EJP, Schalm SW, Van Vliet HHDM. Hemostasis and fibrinolysis in severe liver failure and their relation to hemorrhage. Hepatology. 1986;6(1):79–86. [DOI] [PubMed] [Google Scholar]
- 81.Kovalic AA-O, Majeed CN, Samji NS, Thuluvath PJ, Satapathy SA-O. Systematic review with meta-analysis: abnormalities in the international normalised ratio do not correlate with periprocedural bleeding events among patients with cirrhosis. Aliment Pharmacol Ther. 2020;52(8):1298–1310. [DOI] [PubMed] [Google Scholar]
- 82.Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368(1):11–21. [DOI] [PubMed] [Google Scholar]
- 83.Grabau CM, Crago SF, Hoff LK, et al. Performance standards for therapeutic abdominal paracentesis. Hepatology. 2004;40(2):484–488. [DOI] [PubMed] [Google Scholar]
- 84.Barnett CL, Mladsi D, Vredenburg M, Aggarwal K. Cost estimate of platelet transfusion in the United States for patients with chronic liver disease and associated thrombocytopenia undergoing elective procedures. J Med Econ. 2018;21(8):827–834. [DOI] [PubMed] [Google Scholar]
- 85.Mladsi D, Barnett C, Aggarwal K, Vredenburg M, Dieterich D, Kim R. Cost-effectiveness of avatrombopag for the treatment of thrombocytopenia in patients with chronic liver disease. Clinicoecon Outcomes Res. 2020;12:515–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Terrault NA, Hassanein T, Howell CD, et al. Phase II study of avatrombopag in thrombocytopenic patients with cirrhosis undergoing an elective procedure. J Hepatol. 2014;61(6):1253–1259. [DOI] [PubMed] [Google Scholar]
- 87.Afdhal NH, Giannini EG, Tayyab G, et al. Eltrombopag before procedures in patients with cirrhosis and thrombocytopenia. N Engl J Med. 2012;367(8):716–724. [DOI] [PubMed] [Google Scholar]
- 88.Tateishi R, Seike M, Kudo M, et al. A randomized controlled trial of lusutrombopag in Japanese patients with chronic liver disease undergoing radiofrequency ablation. J Gastroenterol. 2019;54(2):171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lindquist I, Olson SA-O, Li A, et al. The efficacy and safety of thrombopoietin receptor agonists in patients with chronic liver disease undergoing elective procedures: a systematic review and meta-analysis. Platelets. 2021. Epub ahead of print (1369–1635 (Electronic)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Desborough MJR, Kahan BC, Stanworth SJ, Jairath V. Fibrinogen as an independent predictor of mortality in decompensated cirrhosis and bleeding. Hepatology. 2017;65(3):1079–1080. [DOI] [PubMed] [Google Scholar]
- 91.Gluud LL, Klingenberg SL, Langholz E. Tranexamic acid for upper gastrointestinal bleeding. Cochrane Database Syst Rev. 2012;1. [DOI] [PubMed] [Google Scholar]
- 92.Northup PG, Friedman LS, Kamath PS. AGA clinical practice update on surgical risk assessment and perioperative management in cirrhosis: expert review. Clin Gastroenterol Hepatol. 2019;17(4):595–606. [DOI] [PubMed] [Google Scholar]
- 93.Rassi AB, d’Amico EA, Tripodi A, et al. Fresh frozen plasma transfusion in patients with cirrhosis and coagulopathy: Effect on conventional coagulation tests and thrombomodulin-modified thrombin generation. J Hepatol. 2020;72(1):85–94. [DOI] [PubMed] [Google Scholar]
- 94.Sørensen B, Spahn DR, Innerhofer P, Spannagl M, Rossaint R. Clinical review: Prothrombin complex concentrates - evaluation of safety and thrombogenicity. Crit Care. 2011;15(1):201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65(1):310–335. [DOI] [PubMed] [Google Scholar]
- 96.Aldrich SM, Regal RE. Routine use of vitamin k in the treatment of cirrhosis-related coagulopathy: Is it A-O-K? Maybe not, we say. P T. 2019;44(3):131–136. [PMC free article] [PubMed] [Google Scholar]
- 97.Saja MF, Abdo AA, Sanai FM, Shaikh SA, Gader AGMA. The coagulopathy of liver disease: does vitamin K help? Blood Coagul Fibrinolysis. 2013;24(1):10–17. [DOI] [PubMed] [Google Scholar]
- 98.Arshad F, Stoof SCM, Leebeek FWG, et al. Infusion of DDAVP does not improve primary hemostasis in patients with cirrhosis. Blood. 2014;124(21):5044. [DOI] [PubMed] [Google Scholar]
- 99.Kumar M, Ahmad J, Maiwall R, et al. Thromboelastography-guided blood component use in patients with cirrhosis with nonvariceal bleeding: A randomized controlled trial. Hepatology. 2020;71(1):235–246. [DOI] [PubMed] [Google Scholar]
- 100.De Pietri L, Bianchini M, Montalti R, et al. Thrombelastography-guided blood product use before invasive procedures in cirrhosis with severe coagulopathy: A randomized, controlled trial. Hepatology. 2016;63(2):566–573. [DOI] [PubMed] [Google Scholar]
- 101.Rout G, Shalimar, Gunjan D, et al. Thromboelastography-guided blood product transfusion in cirrhosis patients with variceal bleeding: A randomized controlled trial. J Clin Gastroenterol. 2020;54(3):255–262. [DOI] [PubMed] [Google Scholar]
- 102.Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD. Liver biopsy. Hepatology. 2009;49(3):1017–1044. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 103.Bosch J, Thabut D, Albillos A, et al. Recombinant factor VIIa for variceal bleeding in patients with advanced cirrhosis: A randomized, controlled trial. Hepatology. 2008;47(5):1604–1614. [DOI] [PubMed] [Google Scholar]
- 104.Bosch J, Thabut D, Bendtsen F, et al. Recombinant factor VIIa for upper gastrointestinal bleeding in patients with cirrhosis: a randomized, double-blind trial. Gastroenterology. 2004;127(4):1123–1130. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable – no new data generated Data sharing is not applicable to this article as no new data were created or analyzed in this study.


