Figure 4. Xenotransplantation versus Exogenesis — Therapeutic Concepts.
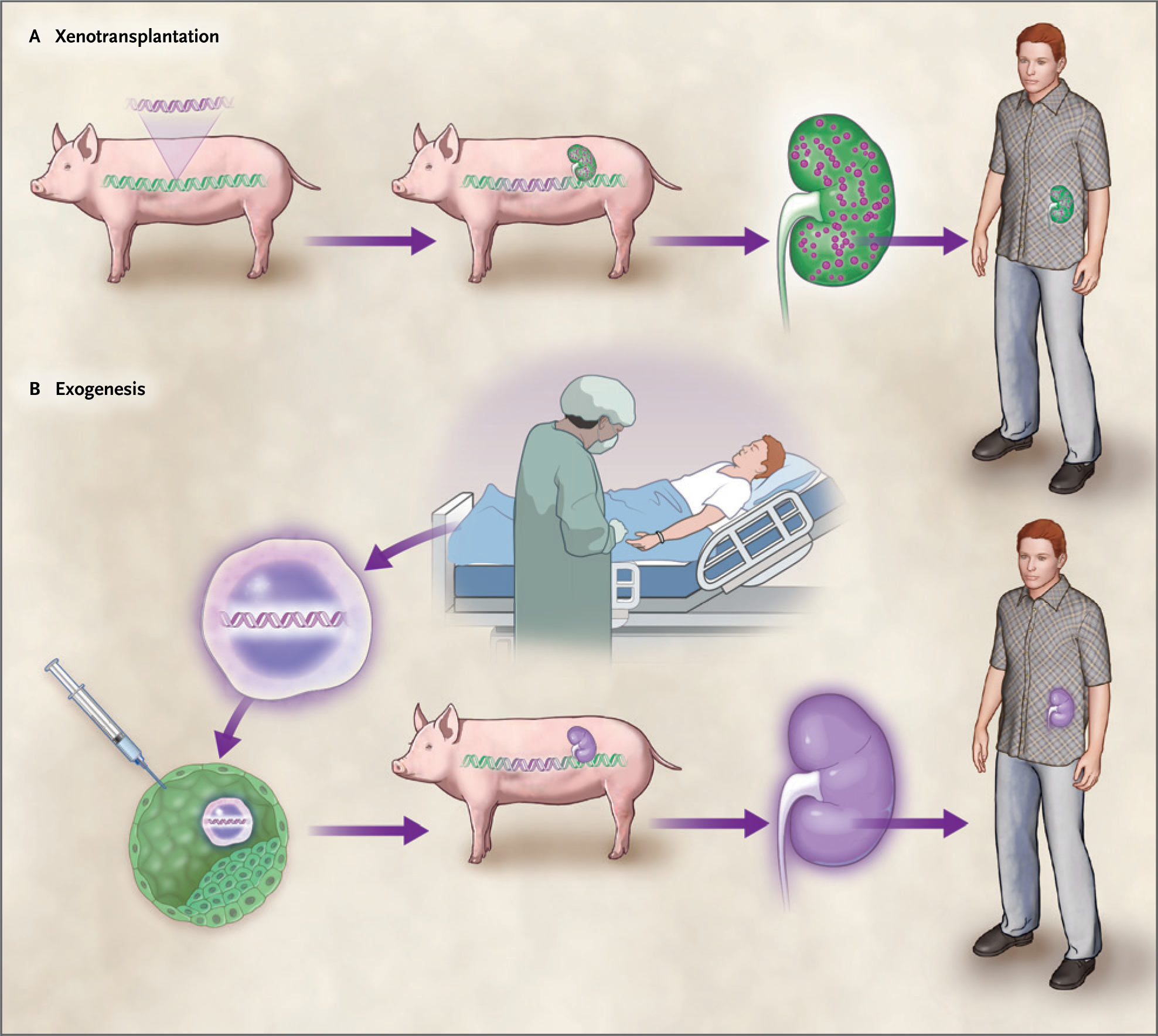
Panel A shows xenotransplantation. (Human components are colored purple, and porcine components are colored green.) The genome of a pig is immunoengineered both by deleting porcine major antigens and by adding specific human transgenes expressing proteins that have been shown or hypothesized to improve the ability of “xeno organs” to persist (e.g., in animal models of xenotransplantation, such as nonhuman primates). Next, the result of the immunoengineering — a donor organism for xenotransplantation — produces an organ modified by immunoengineering. Here, the organ is a kidney, which is green because it has a mostly porcine genome but expresses certain human or humanized proteins (purple circles) intended to improve persistence in humans. Transplantation of the xeno kidney into the recipient restores function. Panel B shows exogenesis, in which a patient is the source of autologous human stem cells (e.g., patient-derived induced pluripotent stem cells [iPSCs]; purple cells). These are injected into a porcine blastocyst derived from a previously immune-engineered pig that has been “humanized” through the deletion of porcine genes and the introduction of human transgenes, as shown in Panel A. The exogenic blastocyst gives rise to a chimera producing a kidney that has a human genome, which can now be transplanted into a recipient.
