FIGURE 6.
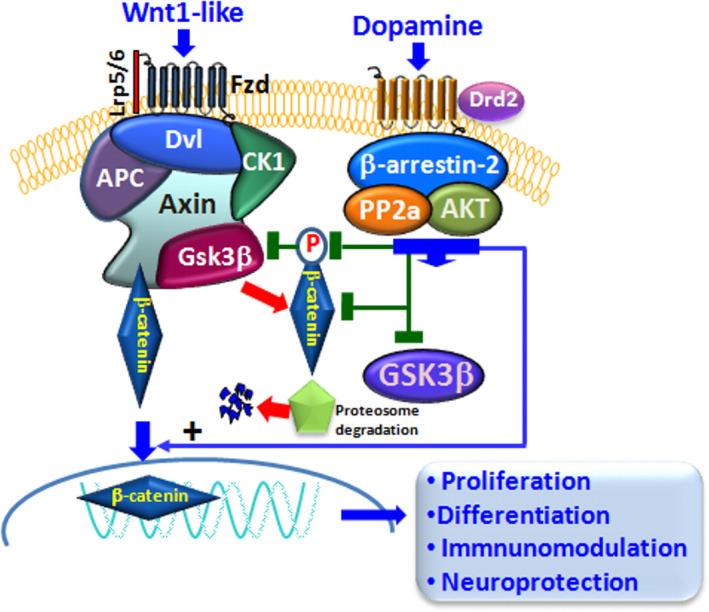
Dopamine signaling pathways crosstalk with Wnt/β‐catenin/GSK‐3β cascade. Simplified representation of the Wnt/β‐catenin signaling pathway and its intersection with DRD2 signaling. Wnt signal activation is tightly controlled by a dynamic signaling complex comprised of core receptors from the Frizzled (Fzds) family of G protein‐coupled receptors (GPCRs), the low‐density lipoprotein (LDL) receptor‐related protein (LRP) 5/6 co‐receptors, and the disheveled (Dvl) and Axin adapters. Binding of Wnt1‐like endogenous/exogenous agonists to Fzd triggers a molecular cascade leading to the cytoplasmic accumulation of β‐catenin, which enters the nucleus, and associates with T‐cell factor/lymphoid enhancer binding factor (TCF/LEF) transcription factors, in turn promoting the transcription of Wnt target genes. β‐Catenin is tightly regulated via phosphorylation by the ‘destruction complex’, consisting of glycogen synthase kinase‐3β (GSK‐3β), casein kinase 1α (CK1α), the scaffold protein Axin, and the tumor suppressor adenomatous polyposis coli (APC). DRD2 downstream intracellular G protein‐independent, arrestin‐dependent pathways can target Wnt/β‐catenin signaling, intersecting GSK‐3β, through the contribution serine/threonine kinase (AKT)‐mediated phosphorylation. Crosstalk between DRD2 and Wnt signaling can relieve β‐catenin from active GSK‐3 phosphorylation, thus permitting β‐catenin translocation in the nucleus activating transcription of Wnt‐dependent genes involved in proliferation, differentiation, neuroprotection and immunomodulation (detailed in the text)
