FIGURE 7.
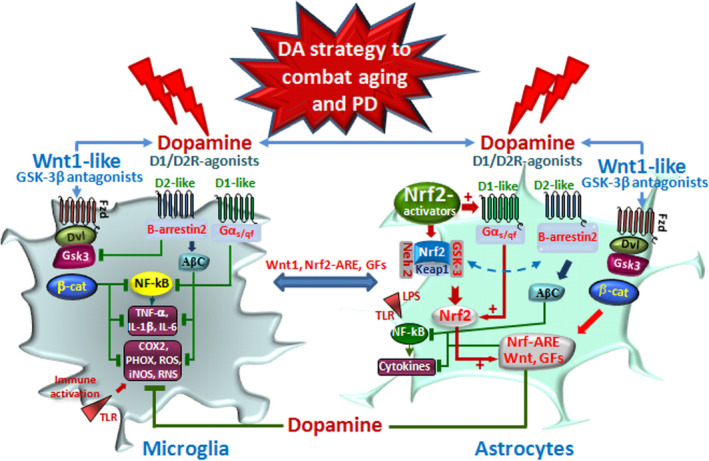
Dopamine drives astrocyte–microglial crosstalk via DRDs/Nrf2/Wnt/GSK‐3 signaling to combat oxidative stress and inflammation. Schematic representation of DA signaling pathways intersecting major oxidative/inflammatory networks in astrocyte–microglial dialogue in PD. Aging, inflammation, and toxic (including bacterial, viral, neurotoxic…) exposures work in synergy with genetic mutations impair nigrostriatal DA neurons. DA and DA agonist can revert such harmful dialogue via a glial switch toward a beneficial antioxidant/anti‐inflammatory and neuroprotective phenotype. DA and DA agonists acting via DRD1 and DRD2 in astrocytes can upregulate Nrf2/HO1 and Wnt1/β‐catenin during oxidative stress and inflammation representing a self‐defense system for mDAn survival. Increased DRD2‐β‐arrestin‐2/AKT cascade may then block GSK‐3β‐induced phosphorylation and proteasomal degradation of the neuronal pool of β‐catenin. Stabilized β‐catenin can translocate into the nucleus and associate with a family of transcription factors and regulate the expression of Wnt target genes involved in DA neuron survival/plasticity, neuroprotection and repair. Oxidative stress engendered by DA itself may also function as a critical negative feedback mechanism via DRD5 induction Nrf2‐ARE cascade and/or via DRD2/β‐arrestin‐2‐induced GSK‐3 inhibition, leading to Nrf2 nuclear translocation. DA‐induced beneficial astrocyte phenotype also intersects microglial inflammatory phenotype via both direct DRD1 and DRD2 transduction pathways inhibiting NLRP3/ NF‐ĸB cascade, and/or via astrocyte beneficial feedback onto microglial cells, via astrocytic Wnt1‐like ligands through Fzd receptors, GSK‐3β antagonist, or HO1‐induced anti‐inflammatory effects
